CHAPTER 24:THE CHEMISTRY OF LIFE: ORGANIC AND BIOLOGICAL CHEMISTRY
General Characteristics of Organic Molecules
The Structures of Organic Molecules
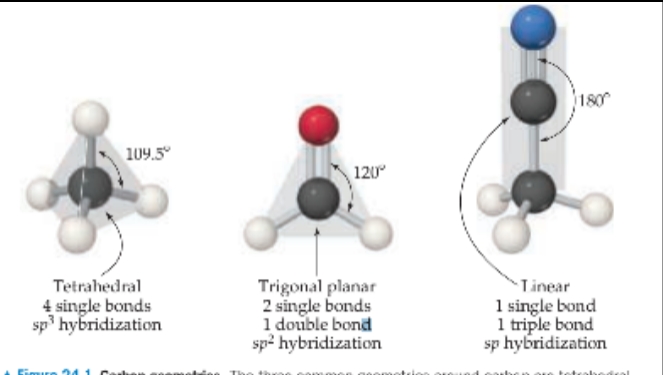
When all four bonds are single bonds, the electron pairs are disposed in a tetrahedral arrangement.
When there is one double bond, the arrangement is trigonal planar.
With a triple bond, it is linear.
The Stability of Organic Compounds
Carbon forms strong bonds with a variety of elements, especially H, O, N, and halogens.
Carbon also has an exceptional ability to bond to itself, forming a variety of molecules made up of chains or rings of carbon atoms.
Most reactions with low or moderate activation energy begin when a region of high electron density in one molecule encounters a region of low electron density in another molecule.
The regions of high electron density may be due to the presence of a multiple bonds or to the more electronegative atom in a polar bond.
[ ] Functional Group — A group of atoms that determines how an organic molecule reacts.
Solubility and Acid-Base Properties of Organic Compounds
- Organic molecules that are soluble in polar solvents are those that have polar groups on the molecule surface, such as glucose and ascorbic acid.
- Organic molecules that are soluble in polar solvents are those that have polar groups on the molecule surface, such as glucose and ascorbic acid function as surfactants and are used in soaps and detergents.
- The nonpolar part of the molecule extends into a nonpolar medium such as grease or oil, and the polar part extends into a polar medium such as water.
- The most important acidic organic substances are the carboxylic acids, which bear the functional group — COOH.
- The most important basic organic substances are amines. \n
Introduction to Hydrocarbons
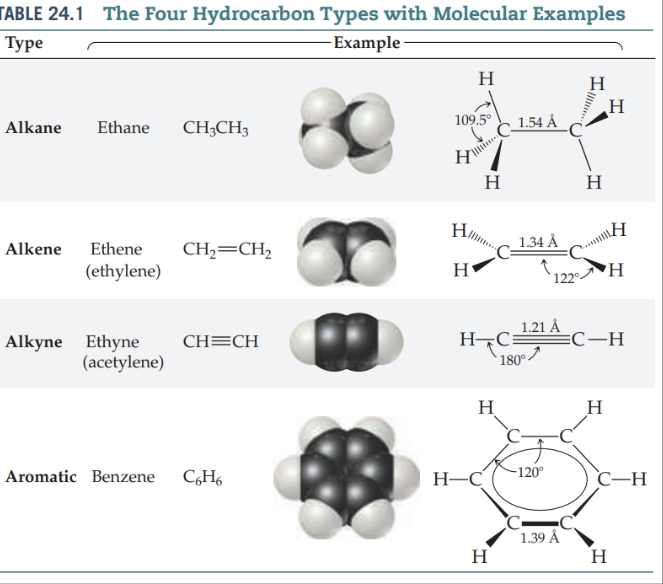
- [ ] Hydrocarbons can be divided into four types, depending on the kinds of carbon– carbon bonds in their molecules.
Alkanes — Contain only single C—C bonds.
Alkenes — also known as olefins, contain at least one C=C double bond.
Alkynes — Contain at least one C𝄙C triple bond.
Aromatic hydrocarbons — Have carbon atoms that are connected in a planar ring structure, joined by both and delocalized p bonds between carbon atoms.
Structure of Alkanes
- According to the VSEPR model, the molecular geometry about each carbon atom in an alkane is tetrahedral.
Structural Isomers
- Structural Isomers — Compounds that have the same molecular formula but different bonding arrangements.
- The alkanes in the table below are called straight-chain or linear hydrocarbons because all the carbon atoms are joined in a continuous chain.
- Alkanes consisting of four or more carbon atoms can also form branched chains, and when they do, they are called branched-chain hydrocarbons.
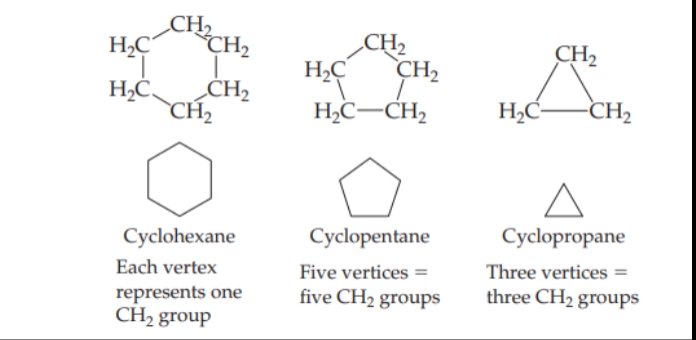
Cycloalkanes
Alkanes that form rings, or cycles.
Alkenes, Alkynes, and Aromatic Hydrocarbons
\n
Alkenes — are unsaturated hydrocarbons that contain at least one C=C bond.
- Ethene — Simplest alkene.
- Geometric Isomers — Compounds that have the same molecular formula and the same groups bonded to one another but differ in the spatial arrangement of these groups.
Alkynes — are unsaturated hydrocarbons containing one or more C𝄙C bonds.
Acetylene – Simplest alkyne.
[ ] Addition Reactions — The most characteristic reactions of alkenes and alkynes, in which a reactant is added to the two atoms that form the multiple bond.
Aromatic Hydrocarbons
Benzene — The simplest and the most important aromatic hydrocarbon
[ ] Substitution Reactions: One hydrogen atom of the molecule is removed and replaced (substituted) by another atom or group of atoms.
Organic Functional Groups
[ ] Alcohols — are compounds in which one or more hydrogens of a parent hydrocarbon have been replaced by the functional group —OH, called either the hydroxyl group or the alcohol group.
[ ] Ethers — Compounds in which two hydrocarbon groups are bonded to one oxygen.
[ ] Carboxylic acids — Contain the carboxyl functional group, often written COOH.
These weak acids are widely distributed in nature and are common in citrus fruits.
[ ] Saponification — The hydrolysis of an ester in the presence of a base.
[ ] Amines — are compounds in which one or more of the hydrogens of ammonia are replaced by an alkyl group.
[ ] Amide — is formed when an amine undergoes a condensation reaction with a carboxylic acid.
Chirality in Organic Chemistry
- [ ] Chiral — A molecule possessing a non-superimposable mirror image.
- [ ] Enantiomers — non-superimposable mirror images.
- [ ] Racemic Mixture — Two enantiomers formed precisely in the same quantity.
\n Introduction to Biochemistry
- [ ] Biochemistry — The chemistry of living organisms.
- [ ] Biopolymers — are polymers produced from natural sources either chemically synthesized from a biological material or entirely biosynthesized by living organisms.
Proteins
These are macromolecules present in all living cells. About 50% of your body’s dry mass is protein.
All proteins are chemically similar, being composed of smaller molecules called amino acids.
Amino Acid — A molecule containing an amine group —NH2, and a carboxylic acid group, —COOH. The building blocks of the protein.
[ ] Peptide Bond — Amide group that is formed by amino acids.
[ ] Polypeptides — are formed when a large number of amino acids 17302 are linked together by peptide bonds.
Protein structure
[ ] Primary Structure — The sequence of amino acids from the “N terminus” along a protein chain.
[ ] Secondary Structure — refers to how segments of the protein chain are oriented in a regular pattern.
Alpha Helix — One of the most important and common secondary structure arrangements; it is held in position by hydrogen bonds between amide H atoms and carbonyl O atoms in the main chain, not the side chains, of the protein.
Beta Sheets — Made of two or more strands of peptides that hydrogen-bond from an amide H in one strand to a carbonyl O in the other strand.
Tertiary Structure — The shape of a protein in its folded form, determined by all the bends, kinks, and sections of rodlike Alpha Helix, Beta Sheets or flexible coil component.
Folding — The process by which the protein adopts its biologically active shape.
Globular proteins — Folded into a compact, roughly spherical shape. They are generally soluble in water and are mobile in cells.
Fibroid Proteins — Forms a second class of proteins; the long coils align more or less in parallel to form long, water-insoluble fibers — providing structural integrity and strength to many kinds of tissue and are the main components of muscle, tendons, and hair.
Quaternary Structure — The way the tertiary subunits are arranged.
Carbohydrates
- [ ] Carbohydrates are a common class of simple organic compounds. A carbohydrate is an aldehyde or a ketone that has additional hydroxyl groups.
- [ ] Monosaccharides — Simple sugars that cannot be broken into smaller molecules by hydrolysis with aqueous acids.
- [ ] Disaccharides — Formed when two monosaccharide units can be linked together by a condensation reaction.
- [ ] Polysaccharides — are made up of many monosaccharide units joined together.
- [ ] Starch — Refers to a group of polysaccharides found in plants. They serve as a major method of food storage in plant seeds and tubers.
- [ ] Glycogen — is a starch-like substance synthesized in the animal body.
- [ ] Cellulose — An important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms.
Lipids
[ ] Lipids — are a diverse class of nonpolar biological molecules used by organisms for longterm energy storage and as elements of biological structures.
[ ] Fats — Lipids derived from glycerol and fatty acids.
Glycerol — An alcohol with three OH groups.
Fatty acids — are carboxylic acids (RCOOH) in which R is a hydrocarbon chain, usually 15 to 19 carbon atoms in length.
Monounsaturated fatty acid — It has only one carbon–carbon double bond in the chain.
Polyunsaturated fatty acid — Have more than one carbon–carbon double bond in the chain.
Phospholipids — are similar in chemical structure to fats but have only two fatty acids attached to a glycerol.
Nucleic Acids
Nucleic acids — are a class of biopolymers that are the chemical carriers of an organism’s genetic information.
Deoxyribonucleic acids (DNAs) — are huge molecules whose molecular weights may range from 6 to 16 million amu.
- Mostly found in the nucleus of a cell.
Ribonucleic acids (RNAs) — are smaller molecules, with molecular weights in the range of 20,000 to 40,000 amu.
- Mostly found at the cytoplasm.
[ ] Nucleotides — The monomers of nucleic acid, formed from a five-carbon sugar, a nitrogen-containing organic base, and a phosphate group.
[ ] The structure of DNA is also the key to understanding protein synthesis, the means by which viruses infect cells, and many other problems of central importance to modern biology.
\n \n \n