CHP207 Human Physiology.docx
CHP207 Human Physiology
Week 1:
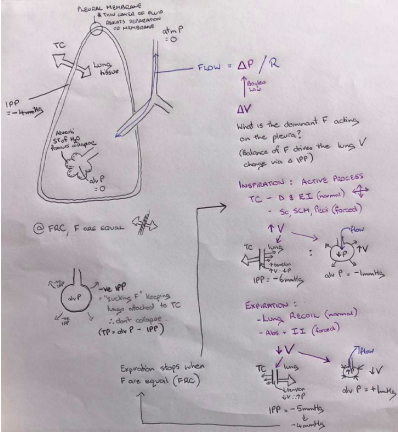
Ventilation and FRC Compliance
Compliance
● Ventilation requires:
○ Change in thoracic volume (P1V1=P2V2)
■ Normal Breathing = active inspiration, passive expiration
- ○ Flow = ΔP/R
- ○ ΔP = ΔV (boyle's law)
- ○ Transpulmonary pressure
- Opening vs collapsing forces
- Difference between alveolar pressure and interpleural pressure (greater
Ptp, greater lung V).
- Visceral membrane stuck to lung/ parietal membrane attached to thoracic
cavity with thin layer of pleural fluid with a negative pressure of -4mmHg
- ○ FRC = when outside pull and inside pull are equal
● Ventilation is affected by: ○ Compliance
- Elasticity of the lung (ΔV/P)
- Surface tension - decreased by surfactant
○ Airway resistance
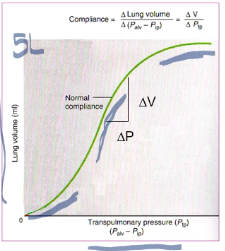
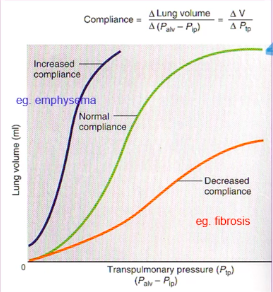
Pulmonary Compliance
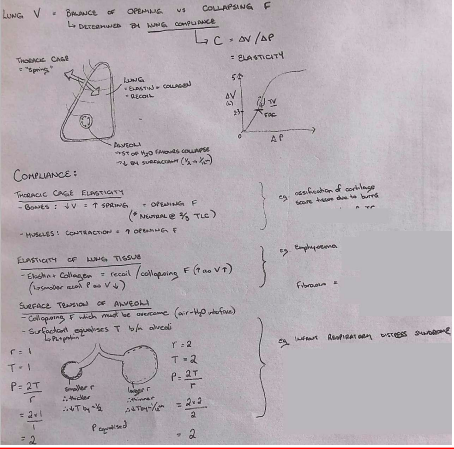
- ● Pulmonary Compliance (C) is a measure of the elasticity of the lungs
- ● It can be represented by the relationship C= ΔV/ΔP
- ● Compliance is generally greatest over the middle range of lung volumes. It is least near minimum and maximum lung
volume
○ Specific compliance = compliance/lung volume
(FRC)
● Lung compliance is affected by:
- ○ Elastic properties of the lung tissue
- Elastin - gives lungs elastic properties
- Collagen - less stretchy but limits over
expansion
- Surface tension of alveolar fluid
- ● In the alveoli, the water surface is trying to contract, and push air out, promoting alveolar collapse.
- ● Therefore surface tension is another force tending to collapse the alveoli, especially towards the end of expiration when alveoli are small
- ● Smaller alveoli= greater P= more likely to empty into larger alveoli and collapse (Laplace' Law) P=2T/r
- ● Surfactant:
- ○ Greatly reduces the surface tension of water
- ○ Secreted by type II alveolar epithelial cells
- ○ Consists of ~85-90% lipids and ~10-15% proteins and
- Surface tension of alveolar fluid
ions.
- ○ It is able to equalize alveolar pressure by varying the
surface tension between alveoli of different sizes
throughout the breathing cycle.
- ○ During inspiration as alveoli expand, surfactant
becomes more thinly spread, increasing surface
tension and decreasing rate of inflation
- ○ During expiration, as alveoli become smaller, there is
more surfactant therefore decrease surface tension
and slowing contraction of the alveoli
- ○ Therefore surfactant, increases compliance as it
reduces surface tension and decreases the amount of
elastic recoil.
- ○ Elastic properties of the thoracic cage
- Twice as much pressure is required to inflate the lungs when they are removed from the thoracic cage
- Thoracic cage reaches the neutral position at ~2⁄3 of vital capacity, after which the direction of its elastic forces
favours expiration. - Affected by:
● Posture ● Obesity- ● Ossification of costal cartilage
- ● Scars resulting from burns to the chest
- ● The greater the lung compliance, the easier it is to expand
the lung at any pressure.
- ● The less compliant the lung, the greater the pressure change
required to expand the lung.
Week 2
Airway Resistance
- ● Flow of air into and out of the lungs is governed by both a pressure gradient (between
mouth and alveoli) and resistance to flow.
- ● Flow=ΔP/R
- ● One of the most important problems in the respiratory passageways is to keep
them open and allow easy passage of air to and from the alveoli ○ Trachea
■ Has cartilage rings, some smooth muscle ○ Bronchi
■ Irregular cartilage plates offer some rigidity and allow sufficient motion for expansion and contraction, smooth muscle.
○ Bronchioles
- Are expanded by the same transpulmonary pressures that expand
the alveoli
- Mainly smooth muscle (except respiratory bronchioles which are
mainly pulmonary epithelium, some fibrous tissue and smooth
muscle fibres).
● Resistance to airflow in the airways
○ **exists only when air is moving
- ● Main non-elastic force governing flow of gas into the lungs is drag/friction in the
respiratory passage (resistance).
- ● Resistance = ΔPressure/Flow (V)
- ● R is influenced mostly by radius of airway
○ Poiseuille’s law: R=8ln/πr(^4)
○ Where l=length, n=gas viscosity, and r=the radius of the tube.
- ● Cross-sectional arTranspulmonary pressure = 2-(-8)=10 of respiratory system
increases from the trachea (2-3cm2) to the alveoli (50-100m(^2))
- ● Resistance is greatest in the first 4-7 branches of the bronchi in comparison to
the number of parallel terminal bronchioles (65 000) which air must pass through
- ● Moving from the single trachea to thousands of respiratory bronchioles increases
the cross sectional area of the airways, therefore the velocity of the air moving through the airways decreases as it travels from the trachea to the periphery of the lung.
- ● Bernoulli principle: The sum of kinetic and potential energy must remain constant. When airflow must remain constant. WHen airflow enters a constriction, linear velocity increases and therefore pressure must decrease (and vice versa).
- ● The main determinants of the cross sectional area (and therefore R) of the airways are:
- ○ Lung volume
- ○ Elastic recoil
- ○ Bronchial smooth muscle tone
- ● Airway resistance changes throughout the breathing cycle/with lung volume
- ● R decreases as V increases
- ○ Small airways distend during inspiration and compress during expiration (ie. also respond to changes in intrapleural pressure)
- ○ Small airways are also attached to alveoli, therefore as alveoli expand, so do small airways.
Elastic Recoil & Dynamic Airway Compression
● R is greatest upon expiration
○ Recoil of the lung during expiration is dependent on the condition of the lung parenchyma
- ● Forced expiration also involves the use of expiratory muscles which may generate a positive intrapleural pressure
- ● Alveolar pressure is high due to high recoil pressure (alveoli have been distended during inspiration) and will decrease as lung volume decreases
- ● A pressure gradient is established through the smaller airways to the mouth
○ Bernoulli principle - P will decrease as we move towards the mouth (a
constriction).
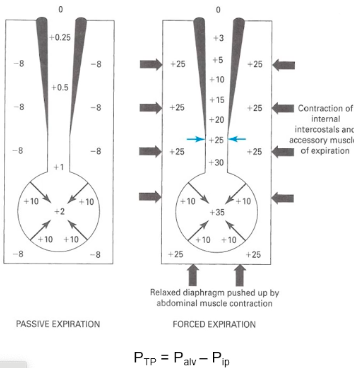
Dynamic Airway Compression ● During expiration:
- ○ 2atm in alveoli
- ○ 0atm in atmosphere
- ○ The natural gradient facilitates passive airflow out
- ○ As we move towards the constriction (large SA in alveoli to small SA in singular trachea)
- ○ Bernoulli principle - The increase in velocity reduces pressure
- ○ ~-8 intrapleural pressure
- ○ P(TP)=P(alv)-P(ip)
- ○ Transpulmonary pressure = 2-(-8)=10
● Forced breathing increases that pressure (from
muscles pushing)
- ○ Intrapleural pressure = 20
- ○ Transpulmonary pressure = 25-(20)=5
- ○ If intrapleural pressure is greater than the
pressure in the airways, compression of the airway may occur
If disease:
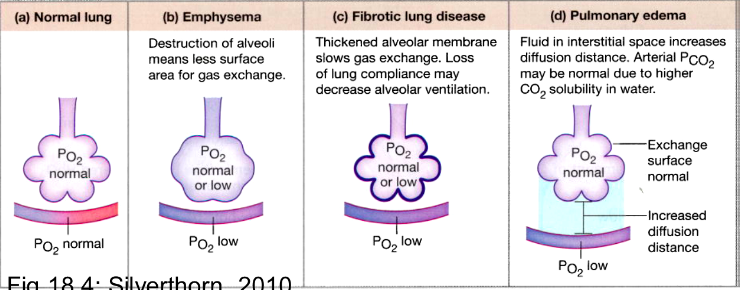
- ● Emphysema:
- ○ Not much recoil
- ○ Need to recruit respiratory muscles
- ○ Airways easier to compress
- ● Fibrosis
- ○ Increase fibrotic scar tissue
- ○ Elastin is replaced by scar tissue
- ○ Protected from airway compression
- ○ Excessive traction
- ● Gas trapping:
○ Can occur in diseased individual which is when gas is trapped in alveoli
because of the increase intrapleural pressures
Bronchial Smooth Muscle Tone
● Smooth muscle in the airways is under control of efferent fibres of the ANS
- ○ Parasympathetic stimulation results in constriction and glandular mucus secretion
- ○ Sympathetic stimulation causes dilation and inhibits glandular secretion
- ○ Histamine and slow reactive substance of anaphylaxis
■ Released by mast cells, cause bronchoconstriction Work of Breathing
- ● 1. The required work to expand the lungs against the lung and chest elastic forces, called compliance or elastic work.
- ● 2. The work required to overcome viscosity of the lung and chest wall structures, called tissue resistance work
- ● 3. The work required to overcome airway resistance to movement of air into the lungs, called airway resistance work.
Week 3:
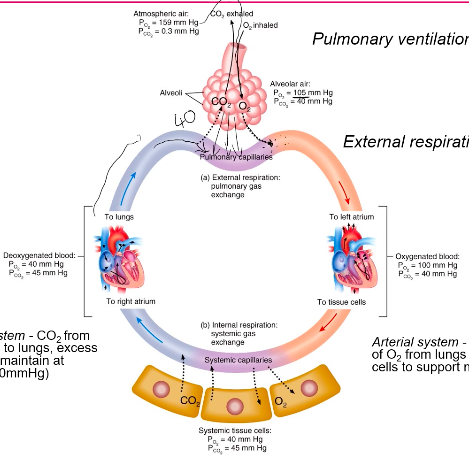
Pulmonary Diffusion & Gas Transport Atmospheric Air
- ● What is the composition of atmospheric air?
- ○ 78.08% Nitrogen
- ○ 20.95% Oxygen
- ○ 0.04% Carbon Dioxide
- ○ 0.001% Water
- ● What is the total atmospheric pressure at sea level? 760mmHg
- ● State Dalton’s law and apply it by calculating the partial pressure of each atmospheric gas at sea level:
- ● states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases:
- ● PTotal=Pgas 1+Pgas 2+Pgas 3...
- ○ 593.408mmHg Nitrogen
- ○ 159.22mmHg Oxygen
- ○ 30.4mmHg Carbon Dioxide
- ○ 0.76mmHg Water
Alveolar Air
The composition of alveolar air is not the same as that of the atmosphere because:
- The atm air we inspire is humidified as it moved through the airways
- ○ Water evaporates from the surface of the respiratory passages and humidifies
the air.
- ○ Vapour pressure at 37 degrees is 47mmHg
- Air in the alveoli is also undergoing continuous exchange with the blood
Therefore the composition of gas exchange as it is inhaled from the atmosphere, through the respiratory passage and to the alveoli.
The alveolar Partial Pressure of Oxygen (ideally 104mmHg) and carbon dioxide (40mmHg) is determined by:
- The level of ventilation
- The level of perfusion
- The rate of diffusion across the membrane
Ventilation (VA) and perfusion (Q) Regional differences in ventilation
- ● Regional differences in ventilation occur due to gravity
- ● Apex:
- ○ Intrapleura; pressure more negative
- ○ Greater transmural pressure gradient
- ○ Alveoli larger, less compliant
- ○ Less ventilation
- ● Base:
- ○ Intrapleural pressure less negative
- ○ Smaller transmural pressure gradient
- ○ Alveoli smaller, more compliant
- ○ Greater ventilation
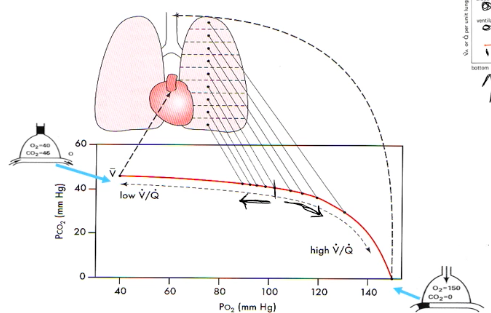
Matching Ventilation and Perfusion (VA/Q)
- ● The level of ventilation and perfusion, and how they are matched is important for efficient
gas exchange and influence alveolar partial pressures.
- ● This matching is discripted in terms of the V/Q ratio.
- ● Considering the lung as a whole, VA and Q are reasonably well matched (VA/Q ratio ~1)
○ Alveolar ventilation of ~5L/min and pulmonary blood flow of ~5L/min ● A mismatched can result in:
- ○ Dead space: the air which does not participate in gas exchange
- ○ Venous admixture: Any blood which passes through the pulmonary circulation
without undergoing gas exchange remains only partially oxygenated. This in effect ‘dilutes’ the oxygenated blood entering the left atrium.
■ In addition, some venous blood from coronary and bronchial circulation is added to the arterial blood diluting it slightly with venous blood (ie. normal anatomical features and venous admixture - slightly similar to dead space).
- ● Thebesian veins - these are part of the coronary circulation. They drain some coronary venous blood directly into the left atrium.
- ● Bronchial circulation - 75-80% of bronchial venous blood discharged into the pulmonary veins.
- ● Together these constitute the ‘venous admixture’ or ‘pulmonary shunt blood.’
- ● VA/Q ratios can range from extremes from:
- ● No VA but normal Q (VA/Q=0), where units contribute to the
venous admixture
- ● Normal VA but no Q (VA/Q=∞), where units become part of the
respiratory dead space.
- ● Most units however would be expected to be within the ‘normal’
category.
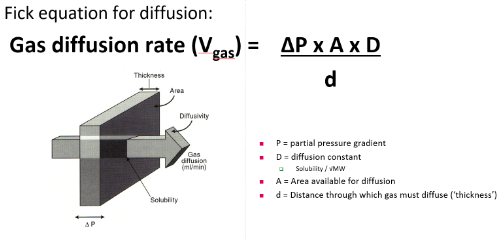
Diffusion across the Respiratory membrane:
- ● 2 anatomical feature that facilitate effective diffusion:
- ○ High SA:V
- ○ High vascularised
- ● The rate of diffusion across the membrane is determined by 5 factors indicated in the
following equation:
●
Anatomical Factors:
- ● Area available for diffusion: this is the surface area of the respiratory membrane.
- ● Distance through which gas must diffuse
Factors relating to respiratory gases:
Partial pressure gradient:
What is the normal partial pressure gradient across the respiratory membrane for PO2 and PCO2?
PO2= 40 mm Hg and a PCO2= 45 mm Hg???
Diffusion constant: CO2 is 23 times more stable than O2, which molecule has a larger molecular weight? CO2
Diffusion Gradients
- ● The movement of gases is dependent on diffusion gradients.
- ● Therefore when gas needs to move from one area to another, it requires a pressure
gradient.
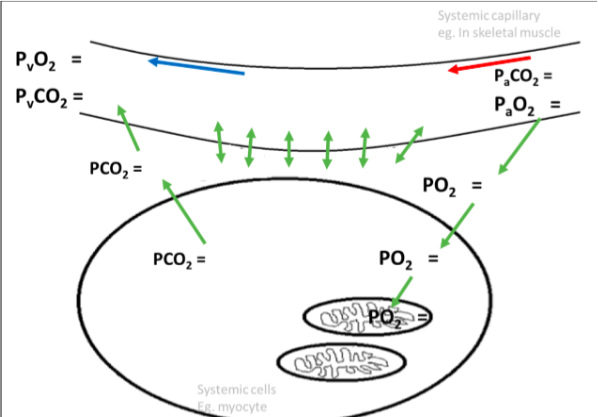
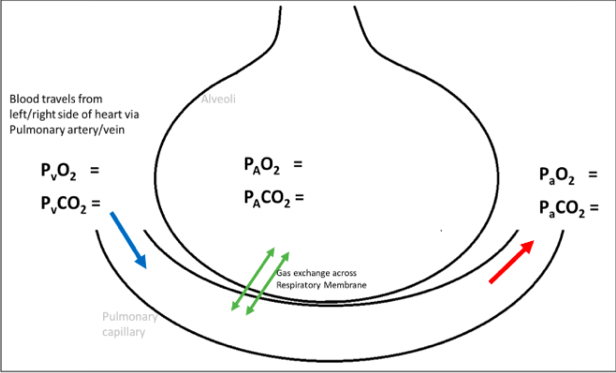
Gas Transport Oxygen
Alveolar air:
Po2 = 105mmHg Pco2 = 40mmHg
Oxygenated Blood: Po2 = 100mmHg Pco2 = 40mmHg
Systemic tissue cells: Po2 = 40mmHg Pco2 = 45mmHg
Deoxygenated blood: Po2 = 40mmHg Pco2 = 45mmHg
Transport of Oxygen
● Only 3% of oxygen travels dissolved in the blood
○ Henry’s law
- ● When we refer to Po2 of the blood, we are referring to dissolved o2***
- ● 97% of oxygen is transported in chemical combination with haemoglobin (Hb):
Hb+O2 -> Hb-O2
- ○ Normal blood contains 150g Hb/L blood
- ○ 1g Hb can bind with upto 1.34ml of O2
- ○ If Hb is fully saturated, 1L of blood carries 200ml of O2.
- ○ At normal resting cardiac output of 5L/min, we can deliver 1000mL of O2/min
(4 times resting O2 requirement).
Haemoglobin (Hb):
- ● There are ~280million Hb molecules per RBC
- ● Moderate size = MW ~64000
- ● Four polypeptide chains
○ Two alpha and two beta chains
- ● Four heme groups containing iron (Fe2+)
- ● Oxygen binds to Fe2+, therefore Hb can carry between 0-4 O2 molecules
- ● Myoglobin: oxygen carrying and storage protein of muscle tissue
- ● There are 4 binding sites on the Hb molecule, the number of oxygen molecules on a
Hb ranges from 0-4
- ● Eg, 98.5% saturation = 98.5% of available sites occupied with O2
- ● Oxyhaemoglobin - red arterial blood
- ● Deoxyhaemoglobin - dark venous blood
○ Pulse oximetry (absorbance of light to determine saturation)
- ● One oxygen molecule reversibly binds to one heme/iron group, therefore one Hb can carry 4 O2.
- ● Haemoglobin exhibits 'cooperative binding’
- ○ When one O2 molecule binds to Hb, it causes a conformational change in the
molecule which increases its affinity for O2, and therefore enhances binding
- ○ THis results in a sigmoid (S) shaped dissociation curve (not linear)
Oxygen-Hb Dissociation curve
- ● PO2 is the main influence on Hb
saturation
- ● At PO2 of 100mmHg, Hb is 97-98%
saturated
- ● At PO2 of 40mmHg, Hb is 75% saturated
- ● At PO2 of 27mmHg, Hb is 50% saturated
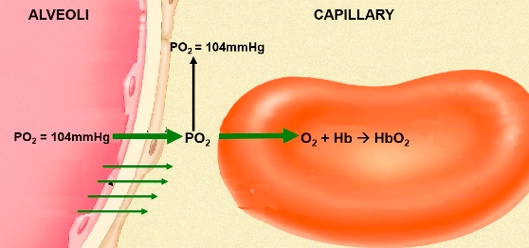
(P50 for Hb) At lungs:
- ● Dissolved O2: blood and alveoli equilibrate PO2 = 104mmHg
- ● At PO2 of 104mmHg, Hb saturation is 98%. At tissue:
- ● Dissolved O2 will equilibrate with tissue, PO2 =40mmHg
- ● At PO2 = 40mmHg, Hb saturation is 75%
- ● Resting o2 requirements = 50ml/L blood
- ● At rest:
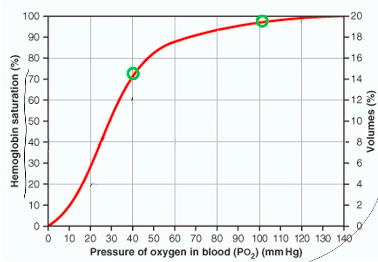
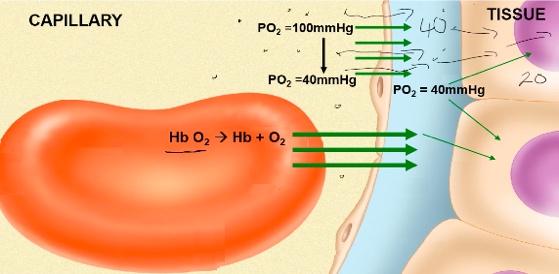
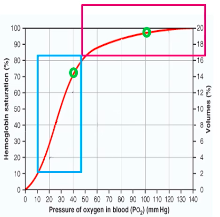
- ● lungs/artery: PO2 = 100mmHg -> 98% Hb saturation -> 200ml/L O2
- ● Tissues: PO2 = 40mmHg -> 75% Hb saturation -> 150ml/L O2
○ 50ml/L O2 delivered to the tissues for a ~60mmHg change in PO2
- ● Binding of O2 with Hb and the rate at which oxygen binds/dissociated (co-operative binding which results in the sigmoid shape of the dissociation curve) means that tissue O2 concentration is held constant over a wide range of alveolar PO2 (at higher PO2).
- ● Flat part of the curve means ~>90% saturation of Hb over a wide range of higher partial pressures (ie, from 60-120mmHg)
- ● Steep part of the curve means large amounts of oxygen can be released from HB with only small changes in PO2, facilitating release into the tissue.
Factors which ‘shift’ the curve
● There are a number of factors which will change the
affinity between O2 and Hb, and therefore shift the curve left/right
○ Shift in the curve will change the P50 ● These include CO2, pH, temperature and
2,3-diphosphoglycerate (2,3 DPG or BPG). These factors facilitate
loading/off-loading of O2 at the lungs or tissue. Bohr Effect: pH and CO2
● At the tissues, the increase in blood CO2 and H+ enhances the release of O2 from the blood into the tissues
○ Decreased affinity -> right shift, increase P50 (increased oxygen off-loading) ● At the lungs, decrease in CO2 and H+ enhances oxygenation
○ Increased affinity -> left shift, decrease P50 (increased oxygen loading)
Hypoxia: low O2 at tissues
- ● Occurs when there is insufficient oxygen available to the cells to maintain adequate
aerobic metabolism
- ● Results in anaerobic metabolism, decrease in pH
- ● Severe hypoxia may result in cyanosis (blue colour - deoxyhaemoglobin)
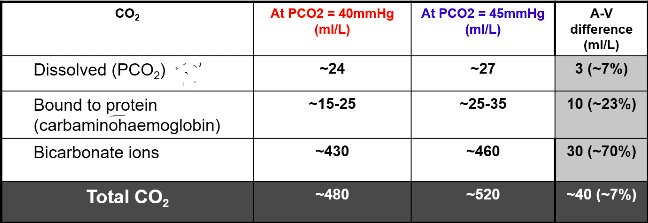
Carbon Dioxide
- ● At rest, tissue metabolism produces ~200-250ml CO2 per min
- ● At cardiac output of 5L/min, we remove ~40-50ml CO2 per L of blood
● We carry CO2 in 3 forms:
CO2 Transport:
● 7% dissolved in plasma
○ It is this form that contributes to PaCO2
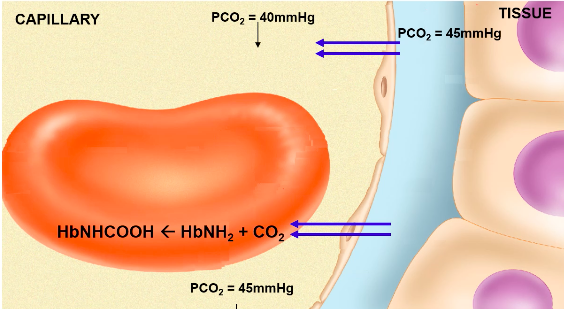
- ● ~10% bound to haemoglobin (protein)
- ○ Reacts with terminal amine group to form carbaminohemoglobin
- ○ Hb-NH2 + CO2 -> Hb-NH-COO- + H+
- ○ Rapid, reversible reaction, no enzyme required
- ○ Reduced Hb can bind more CO2 than oxygenated Hb
- More CO2, offloading at lungs when Hb saturation is increased
- More CO2 loading at tissue when Hb is less saturated
- ● ~80-90% as bicarbonate ion
●
- ● First reaction occurs slowly in plasma, but very quickly in RBC due to presence of
carbonic anhydrase (CA)
- ● H+ is then buffered by Hb or plasma proteins
- ● HCO3- diffuses out of the RBC via a HCO3-/Cl- carrier protein = chloride shift
- ● Reverse reaction occurs at lungs
- ● Hb is a better buffer of H+ ions in reduced state
○ Facilitates CO2 loading at tissues, offloading at lungs
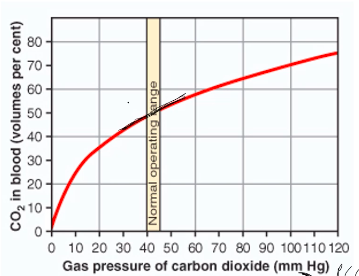
Carbon Dioxide dissociation curve
More linear relationship between PCO2 and CO2 in blood
Much steeper than the oxygen dissociation curve
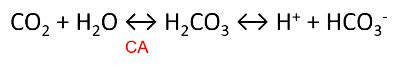
At tissue:
At Lungs:
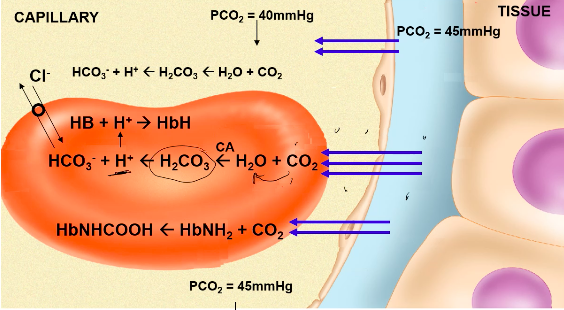
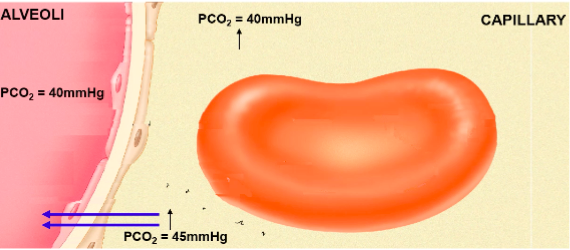
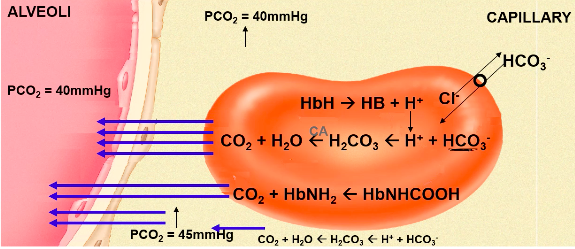
Bohr Effect: pH and CO2
● Bohr effect - how CO2 and H+ affect Hb’s affinity for O2?
○ At the tissues, the increase in blood CO2 and H+ enhances the release of oygen from the blood into the tissues.
■ Decreased affinity -> right shift, increase P50
○ At the lungs, decrease in CO2 and H+ enhances oxygenation
■ Increased affinity -> left shift, decrease P50 ● This occurs because:
- ○ H+ buffered/accepted more readily when Hb is reduced (deoxy-) state
- ○ Increased association of H+ (ie at low pH/high pCO2) with Hb decreases
affinity of Hb for O2. Haladane effect
- ● How oxygen affects Hb affinity for CO2 and H+
- ● The protons produced from the dissociation of
carbonic acid are buffered by Hb
- ● Deoxygenation of Hb enhances its ability to
carry H+ ions
- ● As Hb becomes more deoxygenated it
becomes a more effective buffer -> ‘haldane effect.’
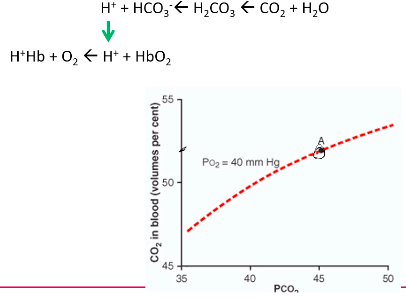
- ● Oxygenation of Hb decreases its ability to carry H+ ions
- ● Therefore at the lung binding of oxygen with Hb releases
excess H+ ions and displaces carbon dioxide
Carbon Dioxide and regulation of acid-base balance
Excretion of CO2 by the lungs is an important mechanism in the regulation of acid-base balance
- ● Changes in PCO2 are a very strong stimulus in the control of breathing
- ● Ventilation will increase 3L/min in for each mmHg rise in PCO2
- ● There is a strong relationship between alveolar ventilation and the concentration of
CO2 in the blood:
●
- ○ Where VA = alveolar ventilation
- ○ K = constant 0.863 (accounts for different units)
- ○ VCO2 = rate of CO2 production
- ○ PaCO2 = arterial PCO2
- ● In healthy people, alveolar PCO2 is in equalibrium with arterial PCO2
- ● Alveolar ventilation tends to be directly proportional to the production of carbon
dioxide (VCO2) and inversely proportional to PCO2.
○ Eg 5L/min = 0.863 x 200ml/min / 40mmHg
Hypercapnia = elevated PaCO2 Hypercapnia may be caused by:
- ● Reduced alveolar ventilation (VA) with normal/constant CO2 production (VCO2)
- ○ Decreased total/minute ventilation
- ○ Increase in dead space
- ○ Due to ventiation pattern (eg, rapid shallow breathing)
- ○ Ventilation/perfusion mismatch
- ● Increased CO2 production without compensatory change in ventilation
- ○ Control problem (suppression of respiratory centre)
- ○ Abnormality of ventilatory pump (severe/advanced emphysema)
Week 4: Ventilation/ Perfusion Matching
● For efficient and adequate gas exchange to occur, ventilation must be matched to perfusion.
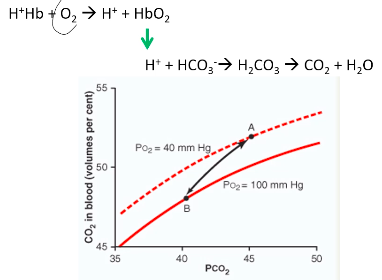
- ● Ventilation is responsible for renewing the partial pressure gradients on the alveolar side of the respiratory membrane (ie. inhaling oxygen rich (PP=~159mmHg), carbon dioxide poor (PP=~0mmHg) atmospheric air, and expiring alveolar air which has undergone gas exchange).
- ● Perfusion is responsible for renewing the partial pressure gradients on the capillary side of the respiratory membrane (ie. bringing venous blood (ie. low oxygen (PP=~40mmHg), high carbon dioxide (PP=~45mmHg) from the venous circulation/right side of the heart).
- ● THe aim of gas exchange at the respiratory membrane is to arterialise the blood ie. increase the partial pressure of oxygen, and maintain constant partial pressure of carbon dioxide (and pH) and ~100mmHg and 40mmHg respectively. In order to achieve this, ventilation and perfusion need to be matched.
V/Q Matching
● In the lungs as a whole, ventilation and perfusion are well matched (ventilation is
~5L/min matched to cardiac output of ~5L/min). However there are regional differences in both ventilation and perfusion which lead to natural regions of mis-match.
Ventilation (V)
● There is a mismatch in ventilation due to gravity and the alveolar being smaller and
tightly packed in the bottom of the lung. This means that ventilation is greatest at the
bottom of the lung due to greater compliance of the alveoli and a larger SA. Perfusion (Q)
● In an upright subject, ventilation increases more slowly than blood flow in the apex of the lung to the base. Hence, the V/Q ratio at the apex of the lung is much greater than 1. Whereas the V/Q ratio at the base of the lung is much less than 1.
V/Q ratio and pulmonary gas exchange Ventilation
● Apex
- ○ Intrapleural pressure more negative
- ○ Greater transmural pressure gradient
- ○ Alveoli large, less compliant
- ○ Less ventilation
● Base
- ○ Intrapleural pressure less negative
- ○ Smaller transmural pressure gradient
- ○ Alveoli small, more compliant
- ○ Greater ventilation
Perfusion
● Apex
- ○ Lower intravascular pressures
- ○ Less recruitment, distention
- ○ Higher resistance
- ○ Less blood flow
● Base
- ○ Greater intravascular pressures
- ○ More recruitment, distension
- ○ Lower resistance
- ○ Greater blood flow
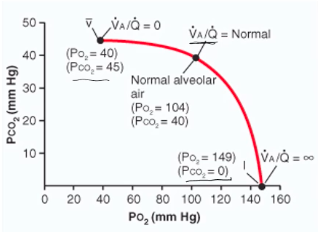
● VA/Q
○ No VA but normal Q (VA/Q=0), where units
ratios can range across extremes from:
contribute to the venous admixture
- ○ Normal VA but no Q (VA/Q=∞), where units
become part of the respiratory dead space.
- ○ Most units however would be expected to be
within the ‘normal’ category.
Regional Differences Alveolar PO2/PCO2
- ● The apex of the lung, being relatively over-ventilated
(under-perfused) has higher PO2 and lower PCO2 than at the base, which is relatively under-ventilated (over-perfused).
- ● In a normal, healthy lung, regional differences in V/Q only have a very small effect on O2 and CO2 concentrations in arterial blood (20.0 and 45 in apex compared to 19.2 and 49 in base).
Effect of Exercise on V/Q ratio
● Exercise, by recruiting more alveoli and more pulmonary capillaries throughout the
whole of the lung, but more especially in the apical regions, produces a smaller range of V/Q ratios.
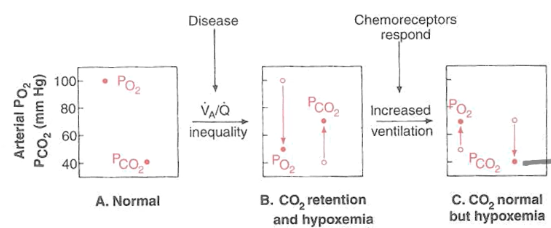
Disease and V/Q mismatch
- ● Non-uniform ventilation may result from:
- ○ Collapsed airway (eg emphysema).
- ○ Bronchoconstriction (eg. asthma).
- ○ Inflammation (eg. bronchitis).
- ○ Compression of airways by tumours, cyst, odema
- ○ Non-uniform compliance (eg. fibrosis or regional variation in surfactant
production).
- ● Non-uniform perfusion may result from:
○ Embolism or thrombosis.
- ○ Compression of pulmonary vessels by high alveolar pressures, tumours, oedema, pneumothorax etc.
- ○ Vascular destruction or occlusion of pulmonary vessels by various diseases.
- ○ Pulmonary vascular hypotension.
- ○ Collapse/overexpansion of alveoli.
EG. V/Q mismatch
● V/Q mismatch and PCO2/PO2 and chemoreceptor response
○
Control of Breathing Medullary Respiratory Centre
- ● The central integrator for breathing control is a system of neurons situated in the brain stem (medulla oblongata and pons).
- ● The medullary respiratory centre consists of a number of groups of neurons:
○ The most important of these are:
■ Dorsal respiratory group (DRG) ● Inspiratory centre
■ Ventral respiratory group (VRG) ● Expiratory centre
● The DRG comprises entirely of inspiratory neurons, whereas the VRG comprises both inspiratory and expiratory neurons.
VRG and forced breathing
- ● Forced breathing involved the VRG and DRG
- ● Recruitment of the VRG inspiratory neurons reinforces those of the DRG during
forced expiration.
- ● Expiration entails not only inhibition of all inspiratory neurons but also activation of
VRG expiratory neurons.
- ● Forced expiration involved contraction of expiratory muscles.
Pontine Respiratory Centre
- ● The pons contains two important control centres that modify the output from the
medullary respiratory centre and fine-tunes respiratory rhythm.
- ● The pneumotaxic centre in the upper pons determines the length of the inspiratory
phase of breathing.
- ○ Increased activity inhibits inspiratory neurons.
- ○ Smooth transition from inspiration to expiration.
● The apneustic centre in the lower pons prolongs inspiratory activity
○ The pneumotaxic centre usually keeps the apneustic centre suppressed.
Factors which alter ventilation
● THere are a number of factors which can influence the respiratory centre and alter
breathing pattern:
- ○ Higher brain centres
- ○ Peripheral chemoreceptors
- Carotid bodies
- Aortic bodies
- ○ Stretch receptors (hering-breuer reflex)
- ○ Irritant receptors
- ○ Proprioceptors in muscles and joints.
Central and Peripheral Chemoreceptors
The chemical control of breathing is one of the main regulators for our ventilatory pattern helping us to achieve our goal of maintaining arterial partial pressures and pH.
● Central chemoreceptors respond to increase in CO2 and H+ (low pH).
○
○ Doesn’t respond to O2.
Aortic Body and Carotid Body:
- ● Made up of glomus cells
- ● If there's not much oxygen in blood, then not much oxygen is being diffused into the
glomus cells.
- ● With this low PO2, the cell begins to depolarise.
- ● Little vesicles that are full of neurotransmitters detect the depolarisation and dump
their neurotransmitters out.
- ● Neurons that are outside the cell are waiting for this signal, this then sends an action
potential down the neuron.
- ● The worse the PO2, the more neurotransmitters being released and the more action
potentials being sent along the neuron.
- ● Similarly, if there's lots of CO2 in blood, not much is being diffused out into the blood
and therefore CO2 builds up in the cell, setting off the vesicles. Additionals points:
● Chemoreceptors only detect/respond to the dissolved for of each gas - ie. PO2 and PCO2 - they do not detect changes in the oxygen and/or carbon dioxide that is bound to Hb.
- ● H+ ions don’t readily cross the blood brain barrier, so the H+ (change in pH) that is detected by the central chemoreceptors is directly as a result of the hydration of CO2.
- ● Changes in arterial pH will be detected by the peripheral chemoreceptors.
- ● A decrease in PCO2 or low H+ (high pH) will also be detected, and stimulate a
response. Essentially the rate of firing from the chemoreceptors will decrease (“quieten”).
Chemical Control of Breathing
● Among the most important inputs to the brainstem control centres originate in the
chemoreceptors
○ Centrally located in the medulla
■ Changes in PCO2, H+
○ Peripherally located in the aortic and carotid bodies
■ Changes in PO2, PCO2, H+ Peripheral Chemoreceptors
- ● Peripheral chemoreceptors are located in or close to the walls of the major arteries in the neck (carotid bodies) and upper thorax (aortic bodies) and receives rich blood supply.
- ● Largest concentration is in the carotid body.
- ● Each carotid body is innervated by a branch of the carotid sinus nerve, which in turn
forms a branch of the glossopharyngeal cranial nerve (IX) which projects to the
medulla.
Peripheral Chemoreceptors: PO2
- ● Carotid body chemoreceptors respond to changes in the partial pressure of oxygen in arterial blood (PaO2).
- ○ There is a small resting discharge from the carotid bodies via glossopharyngeal nerve to medulla at normal arterial PO2.
- ○ When PO2 drops below around 60mmHg, activity sharply increases.
- ○ In hyperoxia, the resting nerve discharge is practically silenced.
- ● Why does PO2 drop <60mmHg before stimulating a significant change in
ventilation?
- ○ This is because of the oxygen, Hb dissociation curve - over a broad range of
PO2, we still have a high range of % saturation and can safely deliver enough
oxygen to cells.
- ○ Our tipping point on the curve is at 60mmHg, from then on, % saturations
drops drastically.
Peripheral Chemoreceptors: PCO2 and H+
● Peripheral chemoreceptors respond to PCO2 in arterial blood.
○ They respond more rapidly than central chemoreceptors, but overall provide
only 25-30% of the total ventilatory response to PCO2.
● Unlike the central chemoreceptors, peripheral chemoreceptors respond to changes in arterial [H+].
○ Eg. ketoacidosis.
● Unlike O2, HCO2 only needs to change by 1mmHg to get a response.
PACO2 and ventilation
- ● The response to an increase in arterial PCO2 over the range from ~35-60mmHg is
linear.
- ● When arterial PCO2 is raised against PO2, absolute ventilation is increased and
there is a significant increase in the slope of ventilatory response to CO2. Chronic effects of Hypercapnia
- ● PCO2 has a great acute stimulatory effect on the respiratory centre.
- ● With chronic exposure to high PCO2, the response gradually declines of 1-2 days to
~1/5th its initial effect due to renal compensation which normalises pH.
- ○ Increased excretion of H+
- ○ Increased reabsorption of HCO3-
- ○ Increased transport of HCO3- across blood brain barrier to buffer H+/
- ● Therefore sensitivity of central chemoreceptors becomes depressed in chronic hypercapnia and response to rises in CO2 is limited.
○ Eg. COPD - increased PCO2, with normal blood pH.
Week 5
Nervous System Membrane Potential
- ● The movement of ions across the membrane creates the “resting membrane potential.”
- ● The resting membrane potential and the imbalance of ions needed to create it are critical to the function of the cell as they allow cells to move other compounds, detect damage, signal to other cells and many other functions.
- ● Potential in the context means voltage.
- ● There is a voltage between the inside and the outside of the cell.
- ● For a neuron, this is about 70mV (context AA battery = x10 neuron voltage).
- ● Voltage: the voltage that exists between A and B is a measure of how much energy it
would take to move a charged particle (eg ion) from point A to point B.
- ● It takes energy to move this charged particle bc there are already lots of charged
particles in that area and there you are going up a concentration gradient.
- ● It requires energy to move negative charges to negative voltages and positive
charges to positive voltages.
- ● The inside of the cell sits at -70mV so that the positive ions outside the cell feel a
force trying to drive them inside the cell.
- ● All ions dont flow in because of the physical membrane that is impermeable to ions.
Potential
- ● Difference in electrical potential energy - aka voltage
- ● Voltage means energy per particle
- ● 1 voltage = 1 joule per coulomb
- ● A coulomb is 6.242x10^18 charged particles.
- ● An electric field is created by charged particles.
- ● A voltage exists when a charged particle is in an electric field.
Potential DIfference
- ● Voltage can only be defined/measured BETWEEN two points
- ● Voltage is analogous to a mass in a gravitational field.
Charged particles can move in a conductor
- ● Current = movement of charged particles
- ● Current is measured in amperes
- ● 1 ampere = 1 coulomb per second
- ● Ohm’sLawV=IxR
- ● V = voltage I = current R = resistance
- ● Resistance is measure in ohms (Ω).
- ● I=V/R
- ○ I = 1.5V/500kΩ (human body resistance)
- ○ I=3uA
- ● Heart:
- ○ I = 1.5V/1kΩ (heart resistance)
- ○ I = 1.5mA
- ○ (this could have physiological impacts)
- ● Defib = 500-1000V
- ● Pacemaker = 2-10V
Important Points:
- ● Potential = voltage = how much energy each charged particle has
- ● Voltage makes charged particles move, aka current.
- ● Conductors have resistance, obey Ohm’s Law, current instantly flows in response to
voltage.
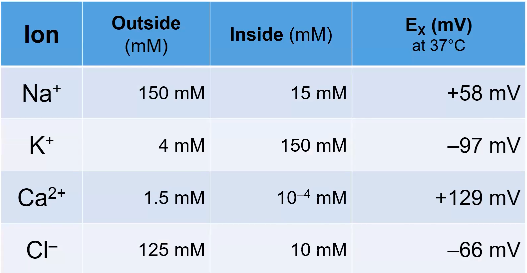
The Equilibrium Potential
- ● Inside all cells are high in K+ ions and low in Na+ ions (130mM and 5mM
respectively).
- ● Outside cells are opposite; low in K+ and high in Na ions (3mM and 130mM
respectively).
- ● Ignoring the effect of resting membrane potential, there is a chemical gradient where K+ want to move out and Na+ want to move in.
- ● The cell membrane is full of K+ ion channels: molecule channels that allow the movement of K+ selectively.
- ● So K+ travels down its conc gradient out of the cell. This movement of K+ out gives the cell a negative voltage ( due to absence of +).
- ● This -tve voltage then applies a tiny bit of force on the K+ outside cell trying to pull it back in but not enough to do anything (channels too strong).
- ● Eventually so much K+ leaves the cell so it becomes super negative and stops the movement of K+ and and therefore no net movement: this is the EQUILIBRIUM POTENTIAL (or nernst potential or reversal potential).
- ● The equilibrium potential for an ion is defined by the concentration of the ion inside the cell and outside the cell but also the charge of the ion and the temp of the cell (at 0 K, the equilibrium potential of all ions is 0mV bc there is no diffusion).
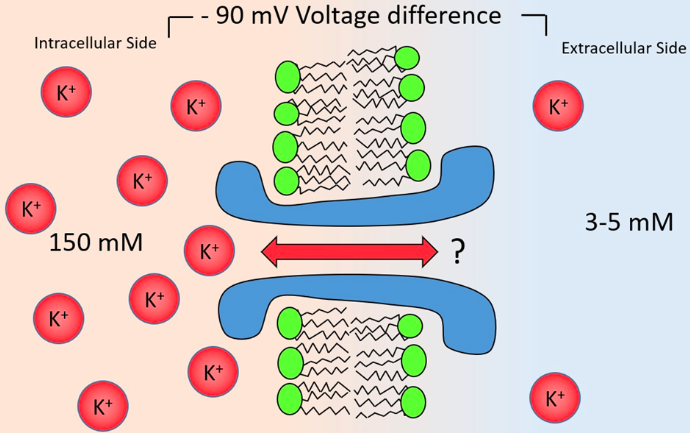
- ● If the membrane potential is at the equilibrium potential for K+, a K+ ion is just as likely to move into the cell as out of it.
- ● The equilibrium potential for K+ is about -90mV, meaning when K+ is allowed to move, it will try to bring the cell to -90mV.
- ● But the resting membrane potential is about -70mV.
- ● Why are resting and equilibrium potential different?
- ○ Because there are other ions at work, eg. Na+.
- ○ There are alot of Na+ outside the cell and little Na+ inside the cell.
- ○ Na+ wants to move inside the cell and it has an equilibrium potential of
+40mV.
- ○ Along with K+ channels, there are also Na+ channels in the membrane, but
far fewer of them.
- ○ Because of this, the cell rests much closer to the equilibrium potential for K+
than Na+.
- ● The equilibrium potential for an ion is calculated with the Nernst equation:
○
- ● Where:
- ○ Ex is the equilibrium potential for ion X
- ○ R is the gas constant (8.3 J mol-1 K-1) - the relationship between energy,
amount of substance and temperature.
- ○ T is the absolute temperature in kelvin
- ○ zisthevalenceoftheion(egCl-is-1,Ca2+is+2)
- ○ F is Faraday’s constant (96,485 C mol-1) - the relationship between the
amount of a substance and its electric charge.
- ● The equilibrium potential is where an ion wants to take the membrane potential to if it
were allowed to flow.
○ To calculate where the membrane potential actually gets to, you need to know
how easily the ion flows, as the bigger a proportion of the total ionic flux an ion makes up, the closer the resting potential will be to that ions equilibrium potential.
The resting membrane potential and Action potentials Electrochemical Gradient
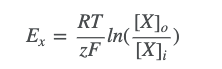
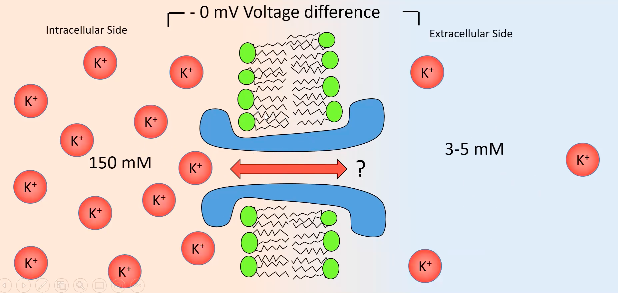
- ● There is no electrical gradient in this instance so this does not have an effect.
- ● There is a chemical gradient and therefore the K+ is going to flow from inside to
outside.
- ● This will then make inside more negative and outside more positive, but not enough
to draw it back in.
- ● As more K+ flows out, inside becomes even more negative, to a point where there is
enough potassium going out to reach equilibrium potential.
Nernst Equation
● At the equilibrium potential for ion X (Ex), the two balance:
- ○ Electrical force felt = tendency to diffuse
- ○ Ie charge x voltage = thermal motion x conc gradient
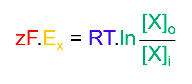
○ ○ ○ ○ ○ ○ ○
●
Z: valence for ion
F: faraday’s constant (96,500 coulombs per mole) R: universal gas constant (8.314 J deg-1 mole-1)
T: absolute temp (kelvins; 0oC = 273K, 37oC = 310K) [X]o = concentration of ions inside cell
[X]i = concentration of ions inside cell
- rearranged formula to find equilibrium potential
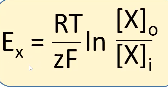
Equilibrium potential:
- ● Electrical force is equal and opposite to chemical “force.”
- ● Diffusion into a cell is equal to diffusion out of the cell.
- ● Equilibrium potential is the potential that that ion is trying to pull the cell to.
Eg:
RT/F = 27 “mV” Potassium: [K+] = 4mM
[K+] = 150mM Z = +1
Hint: think of cells inside sea water, salt is high in water, therefore NaCl is high outside cell.
Calculating Resting membrane Potential
- ● Called the Goldman equation.
- ● Similar to nernst equation but has G terms, G means conductance and is calculated
as the reciprocal of resistance.
- ● Ie. if a membrane lets through a lot of K+ ions, it has a high potassium conductance
and low resistance.
- ● (video version of eq)
●
- ● Think of this equation as a weighted average: the resting membrane potential is the average of all the equilibrium potentials for all the ions in the cell, weighted by how many ion channels of that type are open.
- ○ If mostly K+ channels are open, then the membrane potential is near to the K+ equilibrium potential.
- ○ If mostly Na+ channels are open, then the membrane potential is going to be near to the Na+ equilibrium potential.
- ● Na/K ATPase pump burns energy and pumps K+ from the extracellular space into the cell, and Na+ from inside the cell to out.
Important Points:
- ● Ions will flow down a conc gradient.
- ● Ions will flow in response to voltage (electrical gradient).
- ● The equilibrium potential is the voltage that causes a flow of ions equal and opposite
to the flow due to concentration gradient.
- ● The nernst equation is how you “sum” Nernst equations to calculate a resting
membrane potential of a cell.
- ● Na/K ATPase generates concentration gradients.
- ● Resting membrane potential of neurons is generated by flow of potassium through
ion channels.
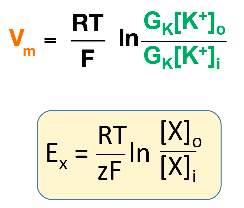
![]()
Action Potential
(H+H = Hodgkin + Huxley)
- ● How neurons signal to each other & how muscle cells synchronise contraction.
- ● If the membrane potential moved to +40mV at the start of the action potential, then it
must be associated with the flow of an ion with an equilibrium potential of +40mV or
above - Na+ (+40mV) and Ca2+ (+120mV).
- ● H+H showed that if you remove Na+ then action potential is blocked but nothing
happens if you remove Ca2+ so therefore action potential must involve Na+.
- ● Similarly, the membrane potential returns to -70mV, therefore there must be a large
increase in the conductance of an ion with an equilibrium of -70mV or lower - K+
(-90mV) and Cl- (-70mV).
- ● When removing K+, action potential destroyed, removing Cl- had no effect, therefore
K+ involved.
- ● DEPOLARISATION PHASE caused by membrane suddenly becoming very
conductive to Na+ ions.
- ● REPOLARIZATION PHASE caused by membrane stopping being permeable to Na+
and become very permeable to K+
What currents cause the action potential?
- ● Na+ influx and K+ efflux
- ● Voltage gated ion channels!
- ● Ion channels can be gated by:
- ○ Chemicals
- ○ Light
- ○ Temperature
- ○ Mechanical Force
- ○ Voltage
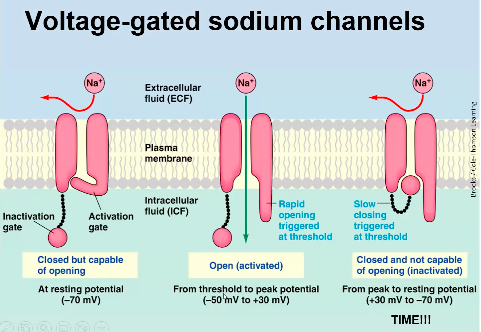
Voltage Gated ion channels:
- ● Something causes the membrane
potential to depolarise a little bit, this then causes a few voltage gated sodium channels to open (activate).
- ● This lets more Na+ into the cell, depolarising the cell more, causing more gates to open and so on and so on...
- ● However, voltage gated sodium channels only remain open for a millisecond or two, as they have an inactivation gate.
○ After being open for a millisecond
or two, the inactivation gate slams shut, putting the gate into an inactivated state.
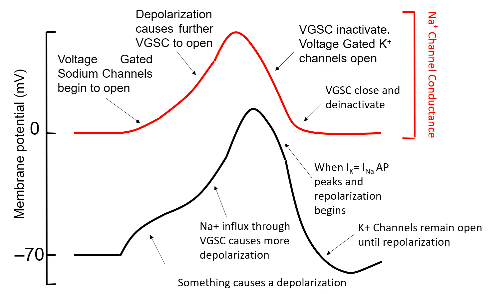
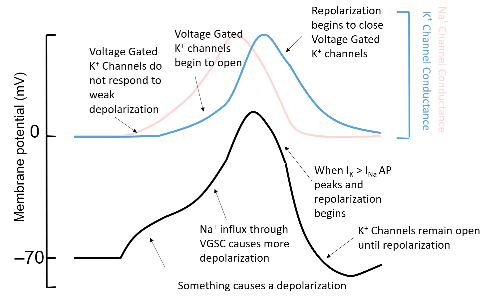
- ○ Once inactivated, the gate cannot open unless it is returned to 079mV where it “deinactivates.”
- ○ So the opening of the the gates pulls membrane potential to +40mV but how does it go down?
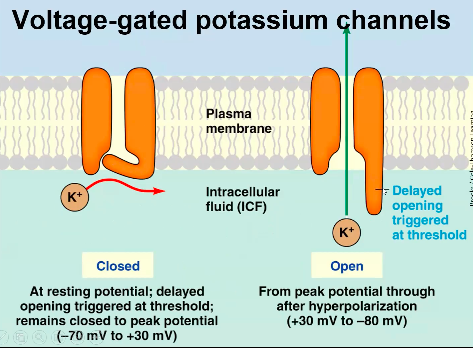
- ● Voltage gated potassium channels then bring the membrane potential back down.
- ● These channels are very similar to sodium ones except for 3 things:
1. They conduct K+ not Na+
2. They do not inactivate: if the cell is more depolarised than about -40mV, these
channels open.
3. Importantly, these channels open more slowly than Na+ channels.
- ● So the action potential beings with something bringing the membrane to a point where voltage gated Na+ channels open and cause a positive feedback loop of depolarisation and opening.
- ● At the same time voltage gated K+ channels begin to open, but too slowly to stop the depolarizing upswing of the action potential, which is terminated by the inactivation of the voltage gated Na+ channels.
- ● At this point, the voltage gated potassium channels are open, pulling the membrane potential back down to -90mV, at which point the voltage gated potassium channels close, and the voltage gated sodium channel deactivates.
- ● This means the cell is now ready to produce another action potential.
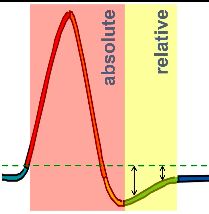
Relative Refractory Period
- ● During the hyperpolarisation which resets channels, the cell is
further than usual from threshold.
- ● So even when the channels are reset, the cell is still more difficult
to excite to threshold.
- ● Until it returns to resting potential, it is relatively refractory - needs
larger than usual stimuli.
Important Points:
- ● Action Potentials begin through positive feedback opening of voltage gated sodium
channels.
- ● APAs are terminated by voltage gated potassium channels,=.
- ● Refractory periods are when it is harder to cause another AP.
- ● APs are propagated down an axon to initiated synaptic transmission.
Week 6 - Nervous System II
Neurotransmission
- ● The primary purpose of action potential is to allow neurons to communicate with
other neurons.
- ● However, the action potential itself does not do the communicating, that role belongs
to the synapse.
- ● The action potential begins where the axon joins the soma, at a place called the
initial segment.
- ● As the action potential begins & Na+ begins rushing in through voltage gated sodium
channels, this not only depolarises the axon initial segment, but it also beings to
weaky depolarise nearby parts of the axon.
- ● Eventually the weaker depolarisation is strong enough to open enough voltage gated
sodium channels to cause the action potential to begin there too.
- ● This in turn depolarises the neighbouring parts of the axon, cause their voltage gated
sodium channels to open, and so on, down the length of the dendrite.
What happens when the action potential gets to the end of the axon?
- ● The axon may split into thousands of fibres, and at the end of these is the
presynaptic terminal.
- ● In the presynaptic terminal there are voltage gated calcium channels.
- ● The depolarisation of the presynaptic terminal causes the voltage gated calcium
channels to open, allowing Ca2+ to enter the presynaptic terminal.
- ● This Ca2+ then binds to proteins on neurotransmitters containing vesicles, causing
them to eject their contents into the synaptic cleft, the small (20nm wide) gap between the presynaptic terminal and the postsynaptic cell.
● The time delay between when Ca2+ influx occurs and synaptic release happens is as brief as 100μs.
● This is notable bc Ca2+ diffuses very slowly within the cell due to the presence of a huge variety of proteins which bind calcium.
● This means that in order for release to happen this quickly, the neurotransmitter containing vesicles must be physically very close to the voltage gated Ca2+ channels.
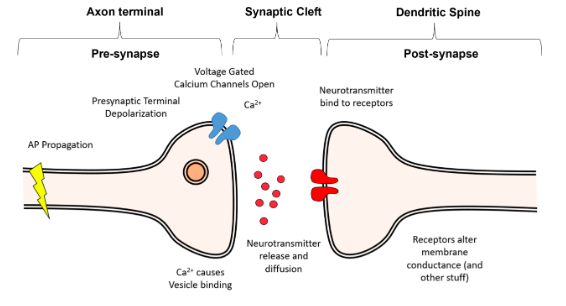
Synaptotagmin - the calcium sensor of the vesicle, once calcium activates synaptotagmin, the process of exocytosis begins.
The SNARE Complex - a complex of proteins which tether the vesicle to the membrane and force it into the membrane to cause release, once synaptotagmin is activated by Ca2+.
● Once released into the synapse, the neurotransmitters simply diffuse across the synapse.
● Diffusion can be very slow over long distances, however, given the minute distance the neurotransmitters have to diffuse over the synapse (~20nm), the diffusion is almost instant on a biological time scale, taking only a few nanoseconds.
A = Action potential
B = Ca2+ entering the cell just after the action potential starts (measured in Amps).
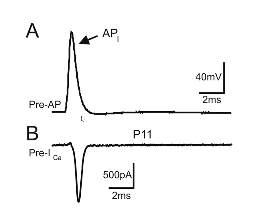
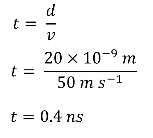
- ● Molecules move fast
- ● RMS speed of neurotransmitters 10-100m/s
- ● Synaptic cleft is about 20nm wide
Overview
- ● Action potentials depolarise presynaptic terminals
- ● Presynaptic depolarisation causes Ca2+ entry through voltage gated Ca channels.
- ● Calcium binds to synaptotagmin to cause vesicle fusion.
- ● Calcium entry is practically restricted to a nanodomain.
- ● Neurotransmitters diffuse across synapse.
Glutamate Neurotransmission
- ● In the mammalian CNS, there are 2 primary neurotransmitters, GABA and Glutamate
(there are approx 100 in total).
- ● Glutamate is the primary excitatory neurotransmitter in the brain.
- ● It is derived from glutamine, and it is chemically identical to the food additive MSG.
Glutamate Receptors
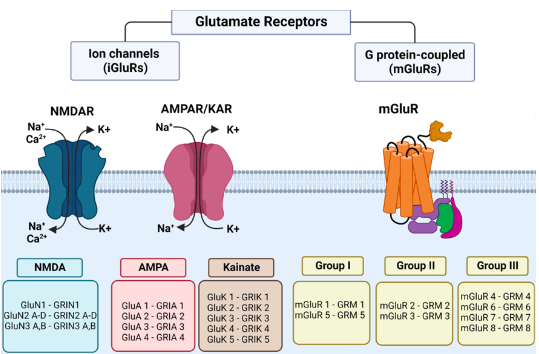
● Glutamate acts on glutamate receptors, which fall into 3 broad classes:
- AMPA/KA receptors - the primary excitatory version.
- NMDA receptors - dependent on both glutamate and membrane depolarisation.
3. Metabotropic glutamate receptors - which commonly have the name mGluR with receptor number.
AMPA/KA Receptors
- ● New receptors used to be identified by their response to drugs:
- ● Two drugs, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic
acid (KA) allowed the identification of two receptors found on almost all neurons.
- ● These receptors are equally permeable to Na+ and K+, and have equilibrium potential
close to 0mV.
- ● They are tetrameric (4 subunits), with AMPA receptors made up of GluA1, GluA2, GluA3
and GluA4 subunits, and typically made up of 2x GluA1 + 2x GluA2 OR 2x GluA2 + 2x
GluA3.
- ● KA receptors are made up of GluK1, GluK2, GluK3, GluK4 and GluK5 subunits.
- ● GluA = AMPA receptors
- ● GluK = KA receptors
NMDA receptors
- ● NMDA receptors were named after their selective ligand, N-Methyl-D-Aspartate.
- ● Similar to AMPA receptors, they are tetramers, made up from GluN1, GluN2A, GluN2B,
GluN2C, GluN2D, GluN3A and GluN3B subunits.
- ● Like AMPA, NMDA receptors are permeable to Na+ and K+ in roughly equal proportion,
and hence have an equilibrium potential near 0mV.
- ● Importantly, they are also permeable to Ca2+ (the permeability of NMDA receptors to
Ca2+ is about 10% that of Na+/K+, so it doesn’t really effect their equilibrium potential
but the permeability is enough to provide a massive source of calcium).
- ● NMDA must bind glutamate in order to open, but they are also voltage gated, and only
become significantly conductive when the cell is depolarised beyond -40mV.
- ● The voltage dependence is produced by Mg2+ ions.
- ● Specifically, Mg2+ wants to enter the cell, much like Ca2+ does, however, the NMDA
receptor is impermeable to Mg2+ and hence it essentially plugs the NMDA receptor.
- ● However, as the cell is depolarised, the Mg2+ is repelled and it leaves the NMDA
receptor, allowing Ca2+ (and Na+/K+) to enter/exit the cell.
- ● NMDA receptors typically exist in the same synapse with AMPA receptors, and so this
allows strong excitatory input to leave a biochemical “footprint.”
- ● Specifically, if the AMPA input is strong enough to depolarise the cell past -40mV, then
Ca2+ will enter the cell, modulating Ca2+ sensitive enzymes/proteins.
Metabotropic Glutamate Receptors (mGluRs)
- ● mGluRs are a collection of 8 different proteins, split into 3 different groups.
- ● mGluRs are not ion channels themselves, but instead activate a g-protein, a small
trimeric protein which splits in two when activated.
- ● THe alpha subunit of the g-protein modulates biochemical cascades, while the
beta/gamma subunits module ion channels.
- ● The 3 different groups of mGluRs are separated by their pharmacology, their synaptic location and finally, which type of g-protein they signal to.
- ● Their actions are hard to find in general, as they typically have different effects in different locations and conditions, though loosely speaking, it can be said that group I mGluRs excite cells, group II mGluRs inhibit cells and group III mGluRs inhibit neurotransmitter release.
GABA Neurotransmission
- ● GABA stands gor gamma-amino butyruc acid, and is a compound derived from the
amino acid glutamine.
- ● It acts on 2 primary classes of receptors, GABAA receptors and GABAB receptors.
GABAA Receptors
- ● GABAA receptors are found almost exclusively on the postsynaptic terminal.
- ● They are ligand gated Cl- channels [meaning they open in response to chemical
binding - in this case chemical GABA].
- ● GABAA receptors are pentamers, meaning they are made up of 5 individual peptides.
- ● These peptides are drawn from 19 possible subunits, each of which gives the final
receptor different properties.
- ● These GABAA receptors with different subunits can be targeted by different drugs
and are often expressed in different locations and drug designers had high hopes for
being able to treat specific diseases by targeting different types of GABAA receptors.
- ● GABAA receptors are Cl- channels, and so when GABA binds to them, the
membrane becomes more permeable to Cl-.
- ● What effect does this have?
○ That depends on the equilibrium potential for Cl- in that cell.
- ○ In some cells the equilibrium potential might be as low as -80mV, meaning that when GABA binds to these neurons, their membrane potential is truly hyperpolarised.
- ○ However, in aot of neurons, the Cl- equilibrium potential is essentially identical to the resting membrane potential of the cell.
- ○ So when GABA binds to GABAA receptors, nothing happens to the resting membrane potential.
● Does this mean that GABA has no action in these neurons?
○ No, It means that if any other source of depolarisation is impacting on the cell,
then the action of the GABAA receptors will work to bring the membrane
potential back to the Cl- equilibrium potential/resting membrane potential.
- ● So in both cases GABA works to prevent sources of depolarisation from bringing the
cell to the AP threshold.
- ● This is why GABA is referred to as an inhibitory neurotransmitter.
GABAB Receptors
- ● GABAB receptors are metabotropic, which means that when they are activated by
their ligand, they activate the G-protein biochemical cascade.
- ● GABAB receptors are found on both pre- and postsynaptic terminals, and their action
differs on where they are located.
- ● Presynaptically, the activity of GABAB receptors leads to the inhibition of voltage
gated calcium channels, meaning that when the action potential invades the presyaptic terminal, less Ca2+ enters the terminal, and hence less neurotransmitter is released.
- ● Postsynaptically, GABAB receptors lead to the opening of potassium channels, which of course hyperpolarises the cell.
- ● Therefore activation of GABAB receptors also has an inhibitory effect.
Neurotransmitter Clearance
- ● Once neurotransmitters are released into the synapse, they eventually diffuse away
from the synaptic cleft.
- ● On this spatial scale, diffusion is raid, but only when there is a strong concentration
gradient, so the concentration of neurotransmitters outside the synapse must be kept
close to 0.
- ● This is achieved either by extracellular enzymes that destroy available
neurotransmitters, or by transporters, which pump the neurotransmitters into a cell.
- ● GABA and glutamate both have neurotransmitter transporters.
- ● The GABA transporter can be found on noth neurons and glial cells but the
transporter of glutamate is particularly noteworthy.
○ Once glutamate is released, it is taken up by glutamate transporters on the
processes of astrocytes (type of glial cell) that surrounds excitatory synapses.
- ○ It seems sensible for this glutamate to be returned back to the neuron that released it, however, the astrocyte can’t simply release glutamate, as this may bind to AMPA/NMDA receptors causing an electrical signal in neurons.
- ○ Instead, the astrocyte converts the glutamate into glutamine which it then releases.
- ○ This glutamine is then taken back up by the neuron, who turns it back into glutamate, before packing it into vesicles, ready to be released.
- ○ This “glutamate-glutamine” cycle seems somewhat complicated, after all, why not just have the neuron take the glutamate up itself.
- ○ However, it has been hypothesised that this allows the astrocyte to take a reading of how active all the synapses in the region are, and then provide appropriate support to the network.
Week 7 - Nervous System III
Coordinated Action of the Nervous System
- ● Responsible for the control of responses to sensory information, control of voluntary
movement and control of involuntary responses which can maintain organ function.
- ● Three types of effectors:
- ○ Somatic - these responses are under the control of the somatic nervous system which control voluntary movement.
- Effector actions include reflexes, actions and behaviours.
- These can be initiated in response to afferent sensory signals that are
- ○ Somatic - these responses are under the control of the somatic nervous system which control voluntary movement.
recived in the brain and activation of the somatic motor system to
initiate a motor action.
- ○ Autonomic - these responses are under the control of the autonomic nervous
system which control involuntary responses.
■ Effector actions include reflexes, coordination and optimisation which
tunes the body to suit certain behaviours or actions.
○ Endocrine - these responses are unto the control of the hypothalamus that
can control endocrine output.
■ Effector actions include systemic control and whole organism action as
a result of secretion of hormones that influence function. Overview of Nervous System:
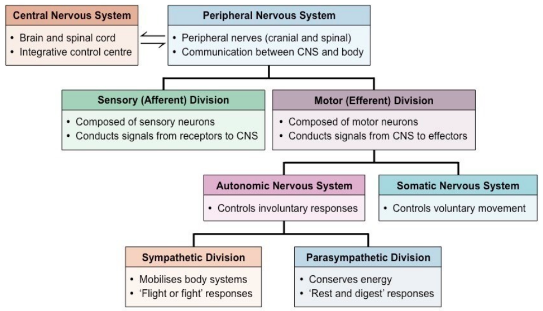
Autonomic Nervous System:
- ● The branch of the motor system that controls involuntary responses important for
both maintenance and activity of specific organs/tissues.
- ● The ANS acts on:
- ○ the heart to modulate heart rate and cardiac output.
- ○ the lungs to modulate respiratory rate
- ○ on blood vessels to modulate blood pressure and blood flow
- ○ And on gastrointestinal tract to modulate digestion.
- ● The ANS has 2 main patterns of physiological regulation:
- ○ Sympathetic Nervous System (SNS) - periods of action and peak
performance.
- ○ Parasympathetic nervous system (PSNS) - periods of maintenance,
conservation and rebuilding.
- ● The dual control optimises the performance of the body to suit its needs.
- ● There are a number of sensory inputs that feed into the ANS, which leads to the
activation of the SNS or the PSNS depending on the circumstances.
- ● There are also a number of autonomic reflexes that enable quick corrective
responses.
- ● The SNS and PSNS divisions of the ANS are anatomically distinct.
- ● The SNS and PSNS often have complementary actions in organs by acting on
cardiac muscle, smooth muscle and glands.
- ● Unlike the somatic motor system which has neuronal cell bodies in the CNS with
heavily myelinated axons that extend in spinal or cranial nerves down to skeletal
muscles, the ANS uses a two-neuron chain to its effectors.
- ● Where the two neurons synapse is called a ganglion.
- ● Both the divisions of the ANS use preganglionic neurons that reside in the brain or
spinal cord and their axons are lightly myelinated thin fibres.
- ● These preganglionic neurons transmit into what's called the autonomic ganglion
which sits outside the CNS.
- ● The postganglionic neurons receives the signal.
- ● The postganglionic neuron then transmits through an unmyelinated thin axon to
effector tissue.
- ● Conduction through the ANS is slower than conduction in the somatic motor system.
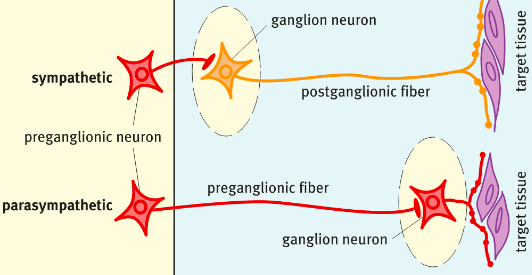
Sympathetic Nervous System (SNS)
- ● The SNS is the action division of the ANS.
- ● It prepares the body for readiness and energy (fight or flight).
- ● It produces a number of physiological actions:
Sympathetic Action | Target Tissue |
Increased HR | Heart |
Increased BP | Blood Vessels |
Relaxes and opens airways | Lungs |
Mobilises energy stores | Muscle and Fat cells |
Increases blood flow to muscles | Blood Vessels |
Stimulates sweating | Sweat glands |
Shunts blood to heart, lungs and brain | Blood vessels |
Suppresses digestion | Gastrointestinal (GI) system |
Relaxes bladder and rectum | Urinary and GI systems |
Action of the SNS on target tissues
- ● To mediate the above actions, the SNS acts on sympathetic ganglia, which leads the
the release of the action hormone adrenaline or noradrenaline directly to tissues,
orchestrating a coordinated response of many organs.
- ● The SNS can also act on the adrenal medulla to secrete adrenaline or noradrenaline
into the bloodstream.
- ● The SNS uses a two-neuron chain to signal to effector tissues.
- ● Preganglionic neurons reside in the spinal cord and are typically short, targeting
ganglia near the CNS.
- ● Preganglionic neurons leave the CNS in ventral roots of spinal nerves T1 to L2 and
synapse in the sympathetic chain ganglia.
- ● Ganglia can be divided into paravertebral ganglia (located near spinal cord) and
prevertebral ganglia (located away from spinal cord).
- ● The paravertebral ganglia are highly interconnected and run the length of the spinal
cord.
- ● The send post-ganglionic fibres in every spinal nerve innervating many effector
tissues such as sweat glands, hair erectors, blood vessels, heart and lungs (stellate
ganglion), salivary glands and pupils (cervical ganglia).
- ● Post ganglionic neurons can often ‘hitchhike’ along major blood vessels to reach
their effector tissue.
- ● Some preganglionic neurons bypass the sympathetic chain and signal to prevertebral ganglia beyond the spinal cord which innervate specific organs such as gastrointestinal system, urinary tracts and genitals.
- ● Some preganglionic neurons bypass both kinds of ganglia and travel to the adrenal medulla, where they synapse on endocrine cells and secrete adrenaline straight into the bloodstream leading to system effects.
- ● Postganglionic neurons are typically long stretching from the spinal column to the innervating organ.
Parasympathetic Nervous System (PSNS)
- ● The is the relaxation and refuelling division of the ANS.
- ● It helps maintain the body during downtime and allows for recovery (rest and digest).
- ● It produces a number of physiological actions:
Parasympathetic Action | Target Tissue |
Decreased HR | Heart |
Decreased BP | Acts via heart rather than directly on vessels |
Closes off airways | Lungs |
Constriction of pupils | Eyes |
Boost digestive system | GI system |
Contracts bladder and rectum | Urinary and GI systems |
Action of PSNS on target tissues:
- ● To mediate the above actions, the PSNS releases the neurotransmitter acetylcholine
(ACh) to target tissues.
- ● Many of the above effects are mediated through multiple ways
○ eg. boosting the digestive system occurs through improving GI smooth muscle activity, GI blood flow, digestive enzyme secretion and GI hormone secretion helping nutrient absorption and use.
- ● Similar to the SNS, the PSNS uses a two-neuron chain to signal to effector tissues.
- ● However there are two sites of outflow from the CNS, the cranial outflow from
brainstem nuclei, and the sacral outflow from the lateral horn of the sacral spinal
cord.
- ● Preganglionic neurons leave the brainstem through cranial nerves 3 (to the eye), 7
(to the lacrimal and salivary glands), 9 (to the parotid gland) and 10 (to the vagus nerve to viscera).
- ● The sacral outflow is through the pelvic splanchnic nerves that travel to visceral ganglia.
- ● Preganglionic parasympathetic fibres from the CNS are very long and travel to parasympathetic ganglia that reside close to or embedded in the target tissue.
- ● As a result, the postganglionic fibres are short and signal direct to tissue.
Neurotransmitters of the ANS
- ● Neurotransmission of the ANS uses two main neurotransmitters, ACh and
adrenaline/noradrenaline.
- ● All preganglionic neurons for both the SNS and PSNS activate ganglion neurons
using ACh on nicotinic ACh receptors.
- ● Ganglion neurons send signals through post-ganglionic fibres to influence target
tissues.
- ● In the SNS, post-ganglionic fibres are adrenergic and release
adrenaline/noradrenaline to act on adrenergic receptors on target tissue.
- ● In the PSNS, post-ganglionic fibres are cholinergic and release ACh to act on
muscarinic receptors on target tissue.
Receptos:
- ● Within the ANS, ACh acts on two types of receptors, nicotinic and muscarinic.
- ● Nicotinic receptors (nAChRs) are ionotropic with four transmembrane domains and
act as ligand-gated ion channels for the cation Na+, K+ and Ca2+.
- ● nAChRs are heteropentamers made up of different subunits and which ions flow
through depend on the subunit makeup.
- ● Activation of nAChRs is excitatory and nAChRs are used by both the SNS and
PSNS at the first synapse.
- ● Alpha motor neurons also release ACh at the neuromuscular junction to act on
nAChRs on skeletal muscle as part of the somatic nervous system.
- ● Muscarinic receptors (mAChRs) are metabotropic and activate or inhibit second messenger pathways within cells to elicit any actions.
- ● They have seven transmembrane domains and are G-protein linked.
- ● There are currently 5 known subtypes that are either Gq-linked or Gi-linked and their
tissue distribution allows them to have different actions.
- ● mAChRs are used by the PSNS at the post-ganglionic synapse and mediate many of
the parasympathetic functions.
- ● Some SNS post-ganglionic synapses use mAChRs such as sweat glands.
- ● Adrenaline (adrenal)/noradrenaline (neural) are used by the SNS only at post-ganglionic synapses.
- ● Following stimulation of nAChRs by ACh at the SNS ganglion, adrenergic fibres release adrenaline to act on adrenergic receptors on the tissue.
- ● There is also a modified sympathetic at the adrenal medulla which acts to release adrenaline into the blood stream.
- ● Adrenaline/noradrenaline acts on adrenergic receptors alpha and beta.
- ● These are metabotropic receptors stimulating second messenger signalling within
cells to elicit their function.
- ● Alpha and beta receptors mediate different function depending on the tissues they
reside in, eg. activation of alpha receptors leads to vasoconstriction, GI shutdown
etc; activation of beta receptors leads to vasodilation, increased cardiac output etc.
- ● There are also subtypes of alpha and beta receptors which can also modulate
function.
Smooth Muscle - the quiet effector cells
- ● Many of the functions that mediate autonomic effects act through smooth muscle
cells.
- ● Unlike skeletal muscle which requires quick contraction and movement, smooth
muscle cells produce a slow, prolonged contraction that makes it an excellent cell for
its homeostatic role.
- ● Smooth muscle controls fluid movement throughout the body as well sphincters to
control access to specific areas of the body.
- ● Activation results in tonic contractions that allow them to support tubes and move
products.
- ● Their slow contractions mean they have low fatiguability and low oxygen use, which
are ideal to maintain key bodily functions.
- ● Smooth muscle is self-stimulating, but hormones and neurotransmitters can
modulate activity.
- ● Smooth muscle cells are small tapered cells rather than long multinucleated fibres of
skeletal muscles.
- ● They have actin and myosin filaments that form a mesh.
- ● Upon contraction, smooth muscle cells bunch up instead of linear pulling like skeletal
muscle.
- ● Smooth muscles cells are not directly controlled by the CNS but create their own
contractile activity via pacemaking: leak channels (K+) which periodically depolarise.
- ● This produces regular cycles of membrane potential, which may or may not cause
actual contractions (Ca2+ influx).
- ● Activity spreads between muscle cells via gap junctions, which are conductive
channels that allow communication between cells.
- ● The role of ANS neurons is to alter pacemaking rate and enhance or inhibit
contraction.
- ● Action potentials are not necessary for smooth muscle cell contraction - calcium
entry is enough.
- ● When contraction occurs, the action potential opens more Ca2+ channels to boost
calcium levels.
- ● The action potentials of a long duration (10-20ms) which is in line with the long slow contraction of the smooth muscle.
- ● The advantage of this is that the contraction can maintain force with very little ongoing activity.
- ● This is ideal for maintaining muscle tone in sphincters, vessels, airways etc.
●
Autonomic reflexes
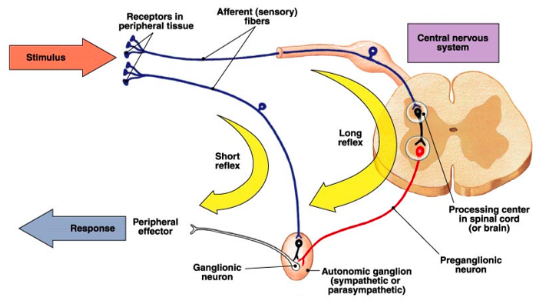
- ● A reflex is a rapid corrective response to a particular sensory event.
- ● The ANS also has reflexes in a similar way that there are somatic reflexes.
- ● These can be classed into 2 types:
- ○ short - where the sensory fibre synapses at the ganglionic neuron (outside the CNS to enact a peripheral response.
- ○ Long - where the sensory fibre reaches the spinal cord or brainstem which activates the pre-ganglionic neuron to signal down to the autonomic ganglion.
- ● Reflexes can be either sympathetic or parasympathetic, depending on the initial stimulus.
● Several CNS nuclei communicate to ANS ganglia to influence autonomic functions in a combined behavioural, autonomic and endocrine response.
● It is the solitary nucleus of the dorsal medulla that receives most of the visceral sensory input from the vagus nerve.
● It then feeds information up to the autonomic integration centres.
● These areas include the parabrachial nuclei (arousal and thermoregulation), the periaqueductal grey (nociception), the hypothalamus (neuroendocrine and physiological functions).
WEEK 8 - Cardiovascular Physiology I
The cardiomyocyte
- ● Cardiomyocytes, or heart muscle cells, are the primary cell type in the heart.
- ● Cardiomyocytes are excitable cells that contract and relax to achieve coordinated
contractions of the atria and ventricles to allow the pumping of blood around the
body.
- ● They work in a manner to enable rhythmic cardiac chamber contractions.
- ● When cardiomyocyte function is impaired or lost - the heart stops working normally
and this has major impacts on whole body physiology.
- ● Cardiomyocytes are physically and electrochemically joined end-on-end at
intercalated disks.
- ● Gap junctions (pores) localise at intercalated disks and allow the propagation of
action potentials from cell-to-cell.
- ● This means an action potential generated in one part of the atria or ventricles can
propagate like a wave throughout the chamber and depolarise all of the cells - this is
called a functional syncytium.
- ● The cardiomyocytes in the atria are physically separated from the cardiomyocytes in
the ventricles by a thick band of fibrous tissue meaning the heart has two functional
syncytia - the atria and the ventricles.
- ● This division in the heart enables atrial depolarisation to occur independently of
ventricular depolarisation.
- ● Atrial and ventricular depolarisations are coordinated by the cardiac conduction
system to enable proper cardiac function. Resting membrane potential in cardiomyocytes
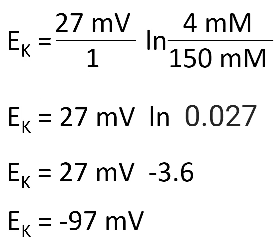
- ● Nernst Equation:
- ○ Where Ex is the equilibrium potential of the ‘X’ ion in mV.
- ○ z is the charge of the ion.
- ○ [X]o is the concentration of the ‘X’ ion outside the cell.
- ○ [X]i is the concentration of the ‘X’ ion inside the cell.
- ● In cardiomyocytes, we really only need to consider 3 ions - sodium (Na+), calcium (Ca2+) and potassium (K+).
- ● Unlike neurons, at rest, cardiomyocytes are almost completely impermeable to Na+ and Ca2+ and are fully permeable to K+.
- ● Therefore, the resting membrane potential for a cardiomyocyte is very close to the Nernst potential for K+.
- ● So if we take K+, where the extracellular concentration in a cardiomyocyte is 4mM and the intracellular concentration is 150mM we have:
●
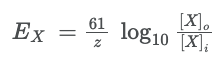
![]()
- ● In reality the resting membrane potential for a normal contractile cardiomyocyte is approximately -90mV. Not exactly -96mV bc it's ever so slightly permeable to Na+ and Ca2+.
- ● This very small contribution of Na+ and Ca2+ shifts the membrane potential only slightly more towards 0mV.
- ● This is a critical point and cardiomyocytes use Na/K-ATPase and Ca2+-ATPase to ensure that intracellular levels of Na+ and Ca2+ are kept low.
- ● Keeping Ca2+ levels low within the cells is critical to ensuring the excitation-contraction coupling can take place.
The cardiac pacemaker cells and spontaneous depolarisation
- ● Cardiac depolarisation does not require input from the CNS.
- ● Cardiac action potentials are spontaneously initiated in collections of specialised
cardiac cells that form pacemakers.
- ● These pacemaker cells generate their own action potentials automatically at regular
intervals (~100/sec).
- ● The SA node is located in the right atria and acts as the physiological pacemaker
under normal healthy conditions.
- ● The AV node is located between the right atria and the ventricles.
- ● The AV node depolarises at a slower rate than the SA node and therefore does not
act as the normal pacemaker.
- ● However, if the SA node is damaged, the AV node can take over as the cardiac
pacemaker albeit with an overall lower rate of depolarisations (leading to lower HR).
- ● The AV node also serves as the conduit from the atria to the ventricles and enables
the transmission of atrial action potentials/depolarisations to the ventricles via the Bundle of His.
Pacemaker cells
- ● 0 - depolarisation
- ● 1 - repolarisation
- ● 2 - plateau
- ● 3 - repolarisation
- ● 4 - resting membrane potential
Phase
- ● ‘Funny’ current activated after preceding repolarisation.
- ● This is a Na channel that causes slow Na influx.
- ● Results in slow depolarisation
- ● In the late part of phase 4 additional Ca2+ channels also
contribute
4 = pacemaker potential
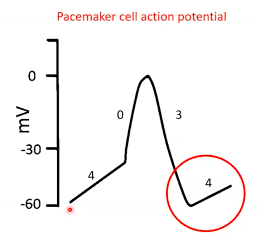
Phase 0 = depolarisation
● Voltage gated Ca2+ channels open at -40mV. ● Ca2+ influx.
● Membrane depolarises.
Phase 3 = repolarisation
● Ca2+ channels close. ● K+ channels open.
● K+ efflux.
● Membrane repolarises.
Summary:
- ● pacemaker cells don't really have a resting membrane potential.
- ● This leaky Na+ channel enables spontaneous depolarisation.
- ● Action potential rate generated by pacemaker cells determines heart rate.
Contractile cardiomyocyte depolarisation and cardiac contraction
- ● Unlike pacemaker cells, contractile cardiomyocytes do not have a leaky Na+ channel
and cannot spontaneously depolarise.
- ● For this reason, contractile cardiomyocytes have a true resting phase where the
membrane voltage is held constant at approx. -90mV.
- ● For contractile cardiomyocytes to depolarise they must be stimulated by an action
potential... which is generated at regular intervals by pacemaker cell depolarisation
in the SA node.
- ● Because all cells in the atria/ventricles are joined at intercalated disks, the action
potential generated by pacemaker cells activates adjacent contractile cardiomyocytes and they continue to spread the action potential like a mexican wave through the atria in a cell-to-cell manner.
- ● This wave of depolarization spreads through the heart and results in a coordinated atrial contraction first, before the signal converges on the AV node and is passed down into the ventricles via the Bundle of His to stimulate ventricular depolarisation/contraction.
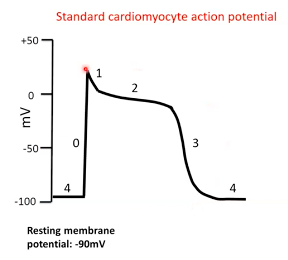
- ● However, the way that a contractile cardiomyocytes depolarises is very different to a pacemaker cell and a contractile cardiomyocyte goes through 5 distinct stages:
- ○ Phase 0 - when threshold is reached (approx. -65mV), voltage gated Na+ channels open rapidly and allow the influx of Na+ and rapid depolarisation.
- ○ Phase 1 - increased concentration of Na+ and positive membrane potential
favours movement of K+ out of the cell.
- ○ Phase 2 - slow acting (L-type) voltage gated Ca2+ channels open and close
over ~200ms. The plateau phase is maintained by a balance between
continues K+ efflux and Ca2+ influx.
- ○ Phase 3 - Ca2+ channels close and additional K+ channels open allowing
greater efflux of K+ and membrane repolarization.
○ Phase 4 - resting phase is re-established, and ion concentrations are rapidly brought back to normal by ion pumps/exchangers.
How do the action potentials cause muscle contraction?
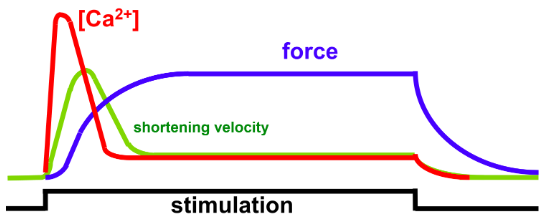
- ● A contractile cardiomyocyte can stay depolarised for a long time - some as long as
300ms.
- ● This is enabled by a long period of Ca2+ influx into the cell that leaves the cell
depolarised (+tve membrane potential).
- ● THis increased time spent depolarised is cruicial as it allows two critical events to
take place within a cardiomyocyte:
- ○ Excitation-contraction coupling
- ○ A refractory period
Video:
Excitation contraction coupling and the refractory period Cardiomyocyte depolarisation
- ● Plateau and repolarisation take a long time compared to skeletal muscle.
- ● Why is this an advantage in the heart?
- ○ 2 main reasons:
- ○ i) Excitation-contraction coupling
- Plateau phase results in prolonged increase in Ca
- Leads to a relatively long contraction
- ○ ii) Refractory period
- A period during which you cannot stimulate another action potential/contraction.
- Avoids multiple action potentials summating like a skeletal muscle (eg. tetanus).
Excitation-contraction coupling (ECC)
The more calcium that is present in the cell, the more powerful the contraction AND The longer the calcium hangs around, the longer the contraction.
Refractory period
● Effective/absolute refractory period
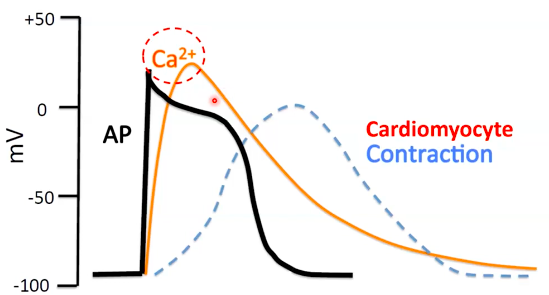
- ○ Na+ channel either all open (phase 0) or are locked (phase 1,2 and some of 3).
- ○ Not possible to generate another action potential.
● Relative refractory period
- ○ Not all Na+ channe;s available
- ○ Greater than norma stimulus is required to generate another AP.
What happens if we try to stimulate more action potential in the refractory period? Tetanic Contractions
● Skeletal muscle
- ○ Summation with higher frequency
stimulation.
- ○ Culminates in tetanus.
- ○ Useful in skeletal muscle.
- ○ Not so much in the heart.
- ○ Prevents the heart from relaxing which
stops the valves from opening and the
heart from filling.
Summary: Refractoriness and non-tetanisation
- ● The cardiomyocyte ‘twitch’ lasts for about 300ms.
- ● The refractory period extends until more than half of the relation period.
- ● This mechanism ensures we get a near complete contraction/ejection of blood
before the heart can contract again.
Control of frequency and force of contraction
- ● At rest, the heart intrinsically generates its own rhythm via pacemaker at
depolarisation.
- ● This results in a sustained depolarisation wave that is conducted through th heart in
a coordinated sequence to generate contraction in an efficient way for blood to be
pumped.
- ● While this happens automatically, the ANS through PNS or SNS activity can modify
the:
- ○ Rate of SA node depolarisation/firing
- ○ Speed of signal conduction, including the time delay at the AV node.
- ○ Force of contraction (SNS only - direct innervation of ventricular heart
muscle).
Video:
Parasympathetic and sympathetic modulation of HR Cardiac Automaticity - signal generation
● Cardiac action potentials are initiated in collections of specialised cardiac cells that form pacemakers.
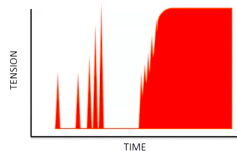
- ● These pacemakers generate action potential automatically at regular intervals.
- ● SA NODE
○ Physiological pacemaker under normal conditions. ● AV NODE
○ Can take over as pacemaker if SA node is damaged.
Multiple autonomic nervous system inputs PNS (vagus)
- ● Parasympathetic nerves innervates mainly:
- ○ SA node (decreases rate)
- ○ AV node (slows conduction and increases AV delay - can completely block it if
signal is strong enough).
SNS
- ● Sympathetic nerves innervates everything:
- ○ SA node (increases rate).
- ○ AV node (speeds conduction, reduces AV delay).
- ○ Conduction network (speeds conduction).
- ○ Ventricular muscle (increases contractility).
PNS - decreasing heart rate
WEEK 9 CV2: control of cardiac output and blood pressure
Cardiac output
- ● The primary function of the heart is to generate and sustain an arterial blood
pressure necessary to provide adequate blood flow to the organs.
- ● The heart achieves this by contracting its muscular walls around a closed chamber
to generate sufficient pressure to propel blood from the left ventricle, through the
aortic valve and into the aorta.
- ● Simultaneously, the right ventricle contracts and propels blood through the
pulmonary valve and into the pulmonary artery to perfuse the lungs.
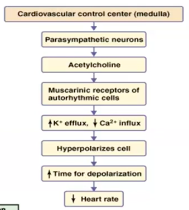
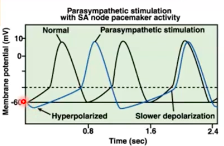
- ● Each time the heart beats, a volume of blood contained within the left and right ventricles is ejected into the aorta and pulmonary artery respectively.
- ● Averaged over time, the amount of blood ejected per beat into the aorta is essentially the same as the volume of blood ejected per beat into the pulmonary artery.
- ● This has to be the case because the cardiovascular system is a closed system - we have ~4.5-5L of blood and this has to be pumped out by the left ventricle into systemic circulation each minute.
- ● For the left ventricle to pump out 4.5-5L, it must receive 4.5-5L of blood from the pulmonary circulation thanks to the work of the right ventricle.
- ● The amount of blood that returns to the right side of the heart from the system circulation, termed venous return, also has to match the cardiac output.
- ● Therefore if cardiac output increases, venous return has to increase to the same extent.
Factors influencing venous return
- ● Venous constriction - constriction of veins (SNS mediated) reduces blood volume in
venous system forcing blood back towards the heart (venous valves prevent
backflow).
- ● Blood volume - an increase in blood volume increases venous return.
- ● Gravity - gravity influence venous blood pressure.
- ● Muscle contraction - contraction of skeletal muscle acts as a pump on veins in
muscles, propelling blood back through the venous system to the heart.
- ● The amount of blood ejected into the pulmonary artery or the aorta each beat is called stroke volume (SV) and is usually measured in mL/beat.
- ● The number of times the heart beats per minute is called heart rate (HR) and is measured as beats/min.
- ● Cardiac output (CO) is therefore the product of SV and HR and is usually measured in mL/min or L/min.
- ● At rest, the average CO is ~4800mL/min
○ This is because average resting HR
is ~60bpm and SV is ~80mL/beat
(60x80=4800)
- ● During intense exercise, CO can increase
to ~35L/min and this is driven by the work/oxygen requirements of working skeletal muscle.
- ● The graph demonstrates the linear relationship between exercise, O2 consumption and CO.
Regulation of HR (and CO)
- ● While SV is a means of increasing CO, HR has a far bigger capacity to
increase from the resting state and therefore can contribute a greater relative
increase in CO when required.
- ● As noted in module 1, resting HR is controlled through the spontaneous
depolarization of the pacemaker cells in the SA node.
- ● However, the parasympathetic (PNS) and sympathetic (SNS) nervous
systems have direct inputs into the heart to regulate HR.
The Bainbridge Reflex
- ● Bainbridge found that infusing blood or saline into the animal via an intravenous line
increased HR.
- ● THis phenomenon occurred even if arterial blood pressure did not increase.
- ● He further observed that HR increased when venous pressure rose high enough to
distend the right atrium, but denervation of the vagus nerve to the heart eliminated
these effects.
- ● This has since been termed the bainbridge reflex.
- ● As venous blood volume increases, the pressure in the vena cava increases.
- ● This results is an increase in the pressure of the right atrium, which stimulates the
arterial stretch receptors.
- ● These receptors in turn signal the medullary control centres to decrease PNS tone
via the vagus nerve to the heart, leading to increased HR.
- ● This reflex ensures that we do not get a build up of blood in the veins/atria.
- ● Summary in my words:
○ Increased venous pressure -> pressure in vena cava increases -> pressure in right atrium increases -> stretch receptors activated -> signal to the medullary control centre to decrease PNS activity -> increased HR.
Regulation of Stroke Volume (and CO)
- ● SV is the volume of blood that is ejected into the pulmonary/systemic circulations
every time the heart beats.
- ● Three primary variables determine SV and therefore CO
○ These are preload, afterload and contractility.
Preload
- ● The amount of blood that fills the ventricles before they contract.
- ● The frank-starling mechanism
- ○ Another intriguing property of cardiac muscle, and indeed most forms of muscle, is that the degree of prior stretch of the myocardium (ie. volume of venous return) influences the subsequent force of contraction (contractility).
- ○ This is an intrinsic property of the muscle and does not require autonomic input.
- ○ This response is the basis of the frank-starling mechanism.
- ○ In simple terms, the higher the amount of ventricular stretch (preload), the
greater the force of the contraction.
- ○ This has the effect of matching venous return to SV (and CO).
Preload continued (video)
- ● In cardiac muscle, the initial length of the muscle sarcomere effects tension
generated in the subsequent contraction.
- ● Increased sarcomere length results in greater tension of the subsequent contraction
(ie stronger contraction). Mechanism of length-tension relationship
● It has been proposed that placing the sarcomere under more tension increases the sensitivity of troponin C to Ca2+.
○ Ie it increases the excitation-contraction coupling efficiency. Preload
- ● In the heart, preload is proportional to the volume of the ventricle at the end of diastole (end diastolic volume [EDV]).
- ● High EDV = increased sarcomere stretch and increases force of contraction.
- ● Practically this means:
- ○ 1. The more blood returns to the heart, the more the heart fills.
- ○ 2. The more the heart fills, the more forcefully it contracts.
- ○ 3. The more forcefully it contracts, the larger the SV (and CO)
- ● This means that the heart has a mechanism whereby if venous return increases, SV and hence CO increases to match - this is called the frank-starling mechanism.
The frank-starling curve
● THis is a ventricular function curve, and clearly shows the frank-starling mechanism
at work.
●
Implications of the Frank-starling mechanism
- ● This mechanism (preload) enables the heart to automatically adjust its own output to match flow requirements in the periphery.
- ● A sudden increase in flow through the tissue results in:
- A rapid increase in venous return to the heart
- The heart responds by increasing the cardiac output to sustain this increased
flow to the tissues.
- Effect of “filling time” on SV
● When HR increases, time available for filling decreases.
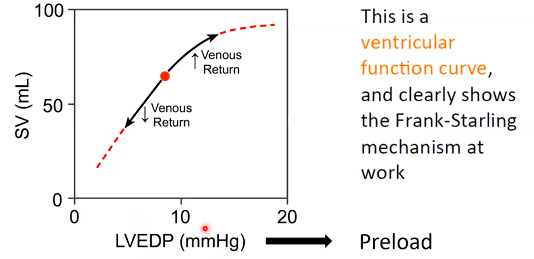
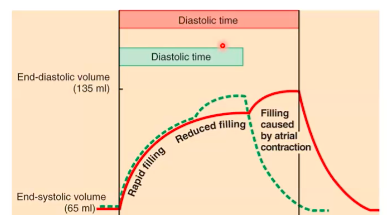
- ● This has little impact with moderate increases in HR as it only affects “reduced filling”.
- ● CO is maintained because any drop in SV is compensated for by increased HR.
- ● As filling time reduces further, the contribution made by atrial systole becomes of
relatively greater importance.
Afterload
- ● Afterload is the force against which the cardiac muscle works during ejection.
- ● Its analogous to the force that a skeletal muscle works against when it shortens.
○ Eg when lifting a weight.
● The afterload of the left ventricle is closely related to aortic pressure.
The ventricles have muscle arranged in roughly a sphere shape
- ● The force that the ventricle opposes when contracting is the wall stress, which is
related to the tension in the ventricular wall.
- ● Therefore, Afterload = wall stress
- ● Wall stress in a pressurised sphere that is described by the Law of LaPlace which
says thats:
Analogy - balloon filled with air
● An increase in radius increases wall stress
Afterload and Cardiac Function
- ● Increasing afterload decreases the contraction velocity of cardiac muscle.
- ● Think about it when you lift a weight:
- ○ Lifting 20 grams is easy and quick
- ○ Lifting 20 Kg is not so easy and relatively slow
- ● Therefore, if afterload increases, less blood is ejected
during each ventricular contraction. The frank-starling curve: effect of afterload
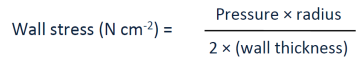
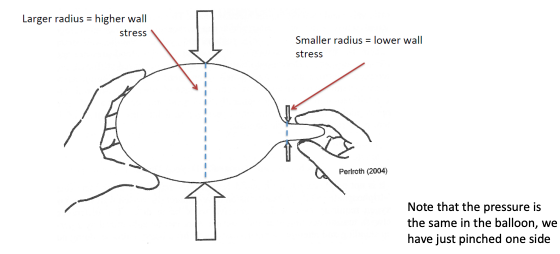
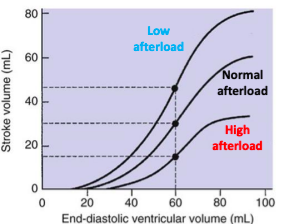
- ● Changing afterload creates a new ventricular function curve.
- ● Increasing afterload results in lower SV at any given preload.
- ● Decreasing afterload results in a higher SV at any given preload.
What can change afterload?
- ● Basically, anything that can change one of these variables:
- ● Systolic pressure of the ventricle:
- ○ Eg increases in hypertension (aortic pressure increased).
- ○ Eg increased in aortic stenosis (ventricle has to generate more pressure to
eject blood).
- ● Radius of the ventricle:
○ Eg. dilated cardiomyopathy ● Wall thickness
○ Eg. ventricular hypertrophy decreases wall stress and is a compensatory mechanism for when afterload increases.
Contractility - The performance of the ventricular myocytes at a given preload and afterload. Contractility of cardiac muscle
- ● Preload and afterload partially depend on the properties of the vasculature (blood volume, resistance, capacitance).
- ● Contractility refers to the rate and amount of force generated by cardiac muscle as it contracts and is independent of either preload or afterload.
- ● Contractility is also called “inotrophy”.
- ● Contractility increases when intracellular Ca2+ influx is increased during plateau
phase/contraction.
- ● Increased Ca2+ influx can occur due to:
- ○ Sympathetic nervous system (SNS) activity.
- ○ Circulating catecholamines (adrenaline).
- ○ Force frequency (faster heart rate produces more powerful contractions).
The Frank-starling curve: effect of contractility
- ● Changing contractility creates a new ventricular
function curve.
- ● Increasing contractility (C) results in higher SV at
any given preload.
- ● Decreasing contractility (B) results in lower SV at
any given preload.
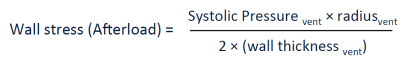
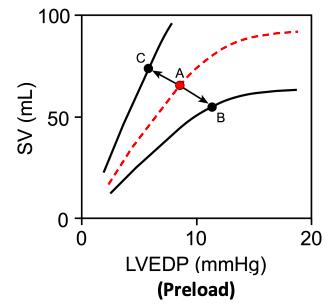
Cardiac output provides arteries with BP
- ● Blood in the cardiovascular system only flows through blood vessels because of the
pressure differences present between the large arteries (high pressure) and large
veins (low pressure).
- ● The heart generates pressure by ejecting blood out with force into the aorta each
time it beats.
- ● The extent of the aortic pressure change depends on the SV.
- ● The aorta typically has the highest blood pressure of all the blood vessels, peaking
at ~120mmHg during systole and dropping down to ~ 80mmHg during diastole in a
resting, healthy individual (this is where the 120/80 mmHg comes from).
- ● From the aorta, blood flows through the arterial system and reaches the small
arteries and arterioles that supply capillary networks within organs
○ These arteries/arterioles are typically called the resistance vessels and
collectively they determine the total peripheral resistance (TPR).
- ● As blood flows through the capillary network and into the venous circulation, the
blood pressure is much lower compared with the arterial system.
- ● As blood reaches the vena cava, blood pressure in this large vein is only ~1-4mmHg
as it enters the right atrium.
Mean arterial blood pressure (MAP)
- ● We use the mean arterial blood pressure (MAP) as the primary indicator of
cardiovascular function and for assessment of organ perfusion.
- ● MAP is determined by the cardiac output (CO), total peripheral resistance (TPR;
sometime also called systemic vascular resistance [SVR]), and central venous pressure (CVP) according to the following relationship, which is based upon the relationship between flow, pressure and resistance (Ohm’s law):
- ● Because CVP is usually at or near 0mmHg, this relationship is often simplified to:
- ● Therefore, changes in either CO or TPR will affect MAP.
- ● In practice, MAP is not determined by knowing the CO and TPR, but rather by direct or indirect measurements of arterial pressure.
- ● From the aortic pressure trace over time, the shape of the pressure trace over time leads to a mean pressure value (geometric mean; ~93mmHg) that is less than the arithmetic
![]()
![]()
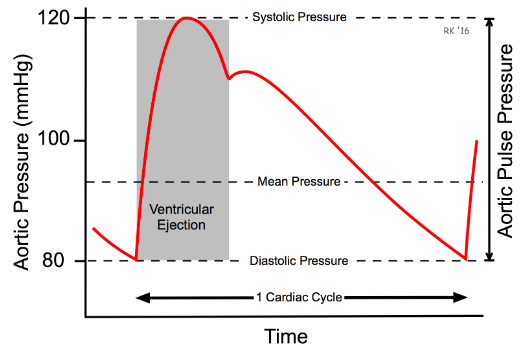
average (100mmHg when time is ignored) of the systolic and diastolic pressures.
- ● At resting HR, MAP can be approximated by the following equation:
- ● For example, if systolic pressure is 120mmHg and diastolic pressure is 80mmHg, then the MAP is approximately 93mmHg using this calculation.
- ● However, at high heart rates, MAP is closer to the arithmetic average of systolic and diastolic pressure (therefore, almost 100mmHg in this example).
- ● This is partly due to the change in CO and also because of the change in shape of the arterial pressure pulse (it becomes narrower due to high HR).
- ● To determine MAP with absolute accuracy, analog electronic circuitry or digital techniques are used.
- ● In normal clinical practice, however, systolic, and diastolic pressures are measured, not MAP.
- ● However, knowledge of systolic and diastolic pressures is sufficient to get a sense of the MAP and thus overall organ perfusion.
- ● Actual measurement of MAP as above can be clinically useful to understand changes in TPR (by rearranging MAP = CO x TPR to TPR = MAP/CO).
Regulation of mean arterial blood pressure (MAP)
- ● MAP is the measurement that explains the average blood pressure in a person’s
blood vessels during a single cardiac cycle.
- ● MAP is significant because it measures the pressure necessary for adequate
perfusion (blood flow) of the organs of the body at any given time (normal range is
between 70 and 100mmHg).
- ● It is considered to be a better indication of organ perfusion than systolic blood
pressure alone (because it also relies on TPR and thus tells us about peripheral
blood flow).
- ● It is vital to have a MAP of at least 60mmHg to provide enough blood to the coronary
arteries, kidneys, and brain to keep them functioning.
- ● A deviation from this range for prolonged periods of time will have drastic
consequences and these will manifest acutely within vital organs. Problems when MAP is too high:
- ● High MAP can cause stress on the heart because it has to work harder than normal to push against the elevated pressure in the vessels (see afterload).
- ● High MAP also causes damage to small vessels (particularly the fragile capillaries) and can lead to advanced heart disease, blood clots, heart attack, and stroke.
- ● Prolonged increases in MAP results in heart enlargement (hypertrophy), which jeopardises the life expectancy of the heart and the patient.
Problems when MAP is too low:
● Low MAP can be life-threatening as well.
- ● When the MAP gets below 60mmHg, vital organs in the body do not get the blood flow they need for survival.
- ● When it gets low, it can lead to shock and eventually death of cells and organ systems.
- ● Low MAP can be caused by sepsis, stroke, haemorrhage/hypovolaemia, or trauma.
- ● For these reasons, the body has numerous inputs into the cardiovascular system to
keep MAP regular at all costs.
Physiological feedback loops - 3 major parts 1. Sensor
○ Detects a change in a physical or chemical property that (eg. vascular stretch in a baroreceptor).
2. Control centre
- ● Integrates sensor signal with appropriate setpoint
3. Effector
- ● Creates a change(s) that opposes original disturbance (eg vascular smooth
muscle relaxation).
Physiological feedback and short-term regulation of blood pressure
- Two main sensors
- ○ The first directly senses blood pressure: stretch in blood vessel walls is sensed by baroreceptors located in various blood vessels (eg. aortic arch).
- ○ The second indirectly senses blood pressure (low perfusion): low oxygen/high CO2 sensed by chemoreceptors in tissues.
- One control centre
○ The vasomotor centre in the brain stem (medulla).
3. Multiple effectors
- ○ Smooth muscle: arteries and arterioles alter total peripheral resistance.
- ○ Smooth muscle: veins and venules change venous return/preload/SV.
- ○ Sino atrial node: SNS and reflex changes in HR.
- ○ Ventricular muscle: SNS changes contractility.
- ○ Adrenal gland: SNS causes release of catecholamines (contractility, SV, HR).
- ○ Kidneys: SNS activates the renin angiotensin system.
Short-term control of MAP Baroreceptors - direct sensors

![]()
- ● These are stretch receptors located in the aorta and carotid arteries
- ● They are tonically active and act to regulate blood pressure from moment-moment
by inhibiting vasoconstriction.
- ● Increased blood pressure -> increases baroreceptor stretch
- ○ This inhibits the vasoconstrictor centre in medulla (less SNS).
- ○ Results in vasodilation, decreased HR and SV.
- ● Decreased blood pressure -> reduced baroreceptor stretch
- ○ Reduced inhibition (ie. more activation) of vasoconstrictor centre (SNS).
- ○ Results in vasoconstriction, increased HR and SV.
- ● Note that there are also different stretch receptors in the atria and pulmonary
systems.
Baroreceptor reflex
- ● A sudden drop in BP decreases baroreceptor firing, which activates the SNS
neurons and inhibits the PNS neurons in the medulla.
- ● The resulting increase in CO and SV act as negative feedback mechanisms to
quickly correct the fall in BP.
Chemoreceptors - indirect sensors
- ● We have peripheral chemoreceptors in aortic and carotid bodies.
- ● We have central chemoreceptors in the brainstem (medulla).
- ● All are stimulated by reduced O2, increased CO2 or pH decrease.
- ● Stimulation of chemoreceptors increases rate and depth of breathing and also
causes peripheral vasoconstriction, increased HR and increased SV.
Chemoreceptor reflex
- Chemoreceptors in the carotid and aortic bodies monitor blood O2, CO2, and pH.
- Chemoreceptors in the medulla oblongata monitor blood CCO2 and pH.
- Decreased blood O2, increased CO2, and decreased pH decrease parasympathetic
stimulation of the heart, which increases the HR.
- Decreased blood O2, increased CO2, and decreased pH increase sympathetic
stimulation of the heart, which increases the HR and SV.
- Decreased blood O2, increased CO2, and decreased pH increase sympathetic
stimulation of blood vessels, which increases vasoconstriction.
Summary:
- ● These physiological feedback mechanisms enable the body to quickly regulate MAP
to ensure organ perfusion.
- ● Baroreceptors respond very quickly to changes in arterial pressure and
activate/deactivate the cardiovascular centre as needed.
- ● Chemoreceptors are slower to respond and appropriately alter CO, TPR in response
to changes in O2 and CO2.
Long-term control of MAP
- ● When we consider the long term control of MAP, this really comes down to regulation
of blood volume.
- ● As noted, our blood volume is typically ~4.5-5L.
- ● When blood volume increases, this extra volume is contained within the
cardiovascular system and we see blood pressure increasing.
- ● When blood volume is reduced, we see less volume within the cardiovascular
system and therefore blood pressure decreases.
- ● Therefore, regulation of blood volume is the same as regulation of blood pressure.
- ● The major system that regulates blood volume (and therefore blood pressure) is the
renal system.
- ● Through the work of the renin angiotensin aldosterone system, the kidneys can
tightly regulate blood volume by controlling excretion rates of Na+ and water in particular.
●
- ● The diagram summarises the main hormones involved in long term blood
volume/pressure homeostasis.
WEEK 10 CV3: Control of blood flow and special properties of the macro- and micro- circulation.
Haemodynamics and control of blood flow
- ● At the end of systole, blood is ejected into the aorta and increases the blood pressure in the aorta.
- ● The generation of pressure by the muscle contraction is the critical factor that determines and drives the movement of blood through the heart chambers and also the entire circulatory system.
- ● How much blood flows, and where it flows to is determined by the metabolic needs of the tissues and organs.
- ● This means that the extent of blood flow to organs is regulated through active constriction/dilation of the arteries that supply these specific organs.
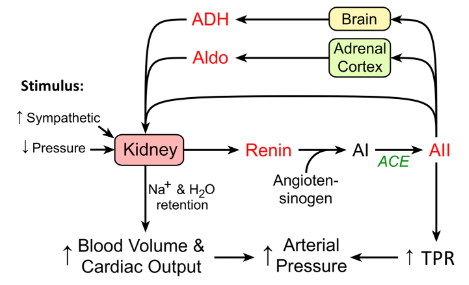
● How constriction and dilation impacts the flow of blood through arteries can be explained by Ohm’s law and Poiseuille’s law that describe the relationship between radius, resistance and flow.
Basic haemodynamics - Ohm’s and Poiseuille’s laws
- ● The laws that govern resistance to blood flow
- ● Ohm’s law:
○ During ventricular systole, blood is ejected from the ventricles at very high pressure.
○ The bulk flow of blood in the cardiovascular system is from high to low pressure.
- ● Poiseuille’s Law:
○ Resistance is:
- Inversely proportional to the 4th power of the vessel radius.
- Directly proportional to the vessel length.
- Directly proportional to blood viscosity. We can combine Ohm’s and Poiseuille’s Laws
The flow through a tube is (pressure difference x radius to the power of 4) divided by (viscosity x tube length).
In the body:
- ● Blood viscosity is related stable
- ● Blood vessels length doesn’t really change
- ● Pressure difference is near enough the same
heart beat-heart beat.
Vessel radius is a very powerful determinant of both resistance and flow
- ● Vessel radius and blood flow
- ○ Only a small change in radius has a massive effect on blood flow
- ○ In the example, changing radius from 1 unit to 4 units increases flow by 256 times (4^4).
- ● Vessel radius and flow velocity
- ○ Increased capacitance (volume).
- ○ Increasing radius also results in increased flow velocity.
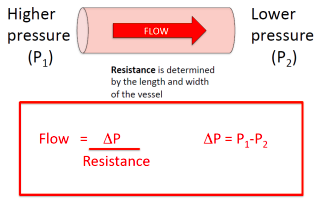

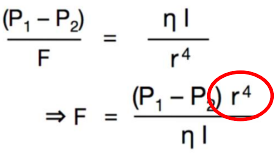
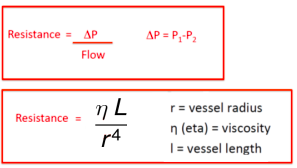
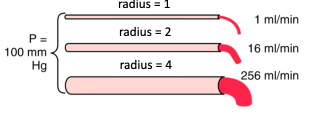
○ The further the blood is located from the vessel wall the faster the flow.
Using the whole circulation as an example
● In this case, the flow is the entire output of the heart =
cardiac output.
- ○ Cardiac output generates the pressure difference.
- ○ Resistance to flow is determined across the entire
peripheral circulation.
- ○ Therefore, Ohm’s law becomes:
Control of blood flow in the body
- ● Not all organs are treated the same by the cardiovascular and nervous systems.
- ● There are essential organs that always get as much blood flow as they need and
use, but dispensable organs that get blood flow when there is enough to go around.
- ● Blood flowing to areas/organs where there is least resistance to flow.
- ● What is the primary determinant of resistance to flow?
○ The radius of the blood vessels it is flowing through.
- ● To recap - when radius increases and specific blood vessels gets more open/dilated,
they can handle an order of magnitude more blood flow because thier resistance to
flow has decreased dramatically.
- ● The radius of blood vessels, in particular arterioles within tissues/organs, controls
where blood flows in the body - these arterioles are often referred to as ‘resistance vessels.’
In the body, six primary factors can modulate the radius of resistance arterioles to regulate how much blood flow they receive:
1. Special receptors
○ Example: skeletal muscle arterioles dilate in response to moderate levels of
adrenaline or insulin. 2. Metabolic control
○ Example: diffusion of metabolic waste products (adenosine, ADP, CO2, lactate, K+ etc) from active cells causes arteriolar vasodilation.
3. Oxygen deficiency (low pO2)
○ Example: oxygen removal from blood by active cells reduces local blood
oxygen levels -> stimulates arteriolar vasodilation. 4. Sympathetic nervous system

○ Example: SNS has direct inputs into most blood vessels and SNS outflow can alter constriction/dilation in arteries and veins to regulate blood flow to individual organs.
5. Nitric oxide
○ Example: shear stress (force exerted by blood on endothelial cells = blood
viscosity x blood velocity/internal diameter) in arteries and arterioles causes their lining endothelial cells to release Endothelium Derived Relaxing Factors (EDRF) like nitric oxide -> nitric oxide is a potent vasodilator.
6. Longer-term control mechanisms
○ Example: capillary density changes (eg. in exercise trained skeletal muscles)
meaning the arterioles need to supply more flow to that tissue -> arteriolar
vasodilation.
Autoregulation and rationing of cardiac output
Regulation of blood flow by metabolism
‘Autoregulation’ of blood flow
● Why is flow not a passive reflection of pressure changes in certain organs?
○ Because some organs require constant blood flow to maintain their functions despite changes is systemic blood pressure
● Two mechanisms are responsible for this in those organs:
- ○ Myogenic mechanism: stretch-induces a reflex in vascular constriction,
prominent in arterioles.
- ○ Metabolic mechanism: metabolic activity of the tissue causes constant
release of vasoactive metabolites and low O2 tension.
Even if systemic arterial pressure changes, blood flow in some organs remains stable
● This is called autoregulation and it is achieved by automatic changes in vascular tone in response to blood pressure.
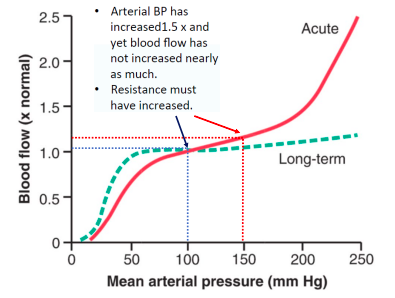
Total systemic blood flow (CO) is the sum of blood flow to all organs
- ● Only if:
- Organ circulations are arranged in parallel with each other AND
- Cardiac output is the sum of all organ blood flows
- ● This parallel arrangement enables us to adjust organ blood flow according to tissue
demands for blood/nutrients.
Sympathetic nervous system innervates the vasculature to regulate in this instance
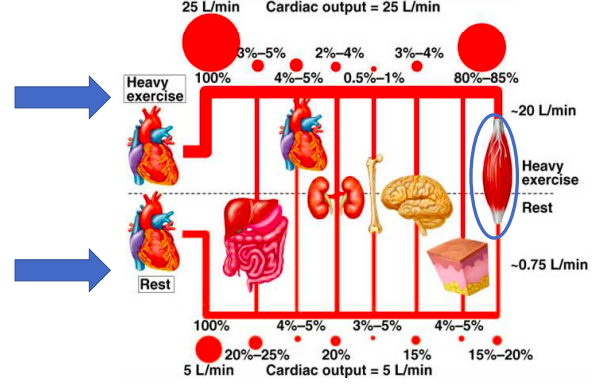
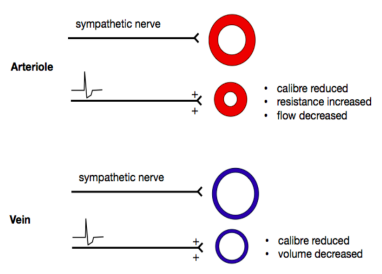
What if cardiac output is limited?
- ● In critical organs (ie. brain, heart, active skeletal muscle) - local flow wins out to
maintain perfusion.
- ● In other organs (skin, gut, kidneys) SNS regulation wins out causing vasoconstriction
and reduced perfusion.
Special properties arteries, veins and capillaries
- ● The major distributing arteries that arise from the aorta
branch into successively smaller arterial vessels until
they become capillaries.
- ● These smallest vessels join to form veins, which then
continue to join with other veins until they enter either the superior or inferior vena cava that bring the blood back into the right atrium of the heart.
Functions of the aorta and large arteries
- ● The aorta, besides being the main vessel to distribute
blood to the systemic arterial system, dampens the pulsatile pressure that results
from the intermittent outflow from the left ventricle.
- ● The actual dampening of the pulsatile blood
flow is a function of the aortic compliance - the ability of the aorta to stretch when it fills with blood during systole and then recoil back to its resting state by actively contracting to continue pushing blood forward.
- ● This recoil is controlled by stretch receptors located in the smooth muscle cells that wrap around the aorta that activate local reflexes to bring cells (and hence the aorta) back to its resting level of tension.
- ● Large arteries branching off the aorta (eg. carotid, mesenteric, renal arteries) distribute the blood flow to specific organs.
- ● These large arteries, although capable of constricting and dilating, serve virtually no role in the regulation of pressure and blood flow under normal physiological conditions.
- ● Once distributing artery reaches the organ to which it supplies blood, it branches into smaller arteries that distribute blood flow within the organ.
- ● These vessels continue to branch and become arterioles.
- ● Together, the small arteries and arterioles represent the primary vessels that are
involved in the regulation of arterial blood pressure as well as blood flow within organs.
- ● Small arteries and arterioles are highly innervated by autonomic nerves (SNS) and respond to changes in nerve activity and circulating hormones by constricting or dilating to regulate blood pressure and blood flow.
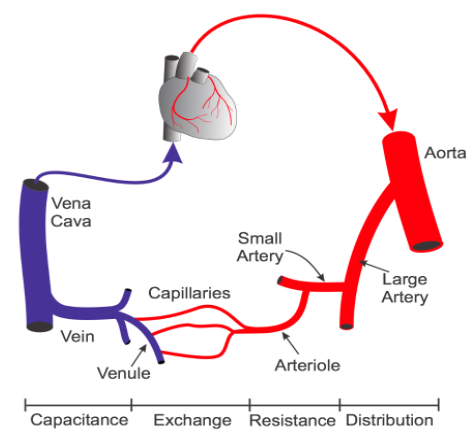
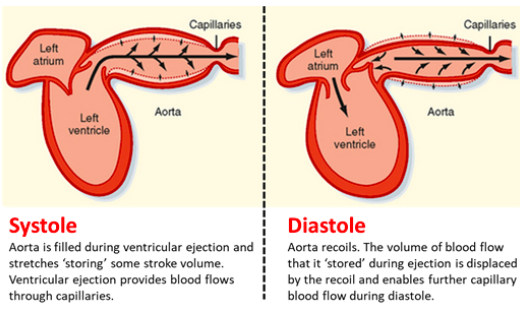
- ● Therefore, these blood vessels are referred to as resistance vessels and as noted, it is these vessels across the body that are largely responsible for generating total peripheral resistance (TPR) and hence play an important role in regulating systemic blood pressure.
- ● As arterioles become smaller in diameter, they lose their smooth muscle.
- ● Vessels that have no smooth muscle but are composed of endothelial cells and a
basement membrane, are termed capillaries.
Functions of the capillary network
● Capillaries represent the smallest vessels (~5-10um in diameter) within the
microcirculation and arguably perform the most important role of the cardiovascular systems:
○ They facilitate nutrient exchange between blood and tissues they supply.
- ● Terminal arterioles can divide and give rise to as many as 15 capillaries.
- ● The motor-regulating mechanism of capillaries outlines that terminal arteriolar
constriction/dilation controls capillary flow.
- ● To this end, dilation of the arteriole was proposed to increase blood flow through
each downstream capillary equally.
- ● Over the last 10 years, advanced microscopy methods have revealed that capillaries
themselves have cells wrapped around them that can constrict/dilate individual capillaries - these cells are called pericytes and they are similar to smooth muscle cells.
- ● These new discoveries indicate that blood flow (and hence blood pressure) in individual capillaries can be regulated so that individual cells that the capillary supplies receive just enough blood flow (and hence nutrients) at any given time.
- ● What controls these mechanisms and how these very finely tuned regulation of capillary blood flow changes in disease states remains unknown.
- ● Across the capillary endothelium, oxygen, carbon dioxide, water, electrolytes, proteins, metabolic substrates, and by-products (eg. glucose, amino acids, lactic acid), and circulating hormones are exchanged between the plasma and the tissue interstitium surrounding the capillary.
- ● While all capillaries have similar functions, their composition differs between different organs.
- ○ Continuous capillaries have very tight junctions between endothelial cells and tightly regulate what passes out of the blood. Most organs have these sort of capillaries with some small variations.
- ○ Sinusoidal capillaries have large gaps between endothelial cells and lots of substances can easily pass through into the tissue. Only the liver has this sort of capillary structure.
- ○ Fenestrated capillaries have tight junctions but the endothelial cells have small holes/pores in them and allow small-medium sized substances to pass
through. The kidneys are the primary site in the body where these capillaries are found.
Starlings forces and capillary filtration
Movement of solutes or filtration across capillaries depends on 2 things:
1. The balance of hydrostatic and osmotic pressure across the capillary
○ This is called the Net Filtration Pressure (NFP) = total pressure promoting
fluid movement to/from capillary
2. The surface area and permeability coefficient (Kf) of the capillary
Starling’s forces: pushing fluid in and out of capillaries
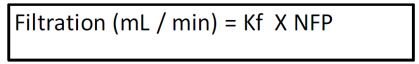
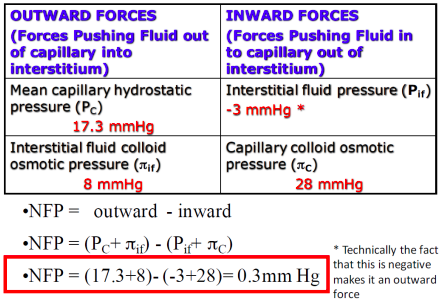
So does that mean very little fluid flow to or from capillaries?
Yes and No
Yes: The average capillary hydrostatic pressure is ~17.3mmHg. Therefore, there is not much net movement of fluid to and from the interstitial space.
No: The arterial end of the capillary is at higher pressure than 17.3
The venous end of the capillary is at lower pressure than 17.3
Blood flows from high-low pressure
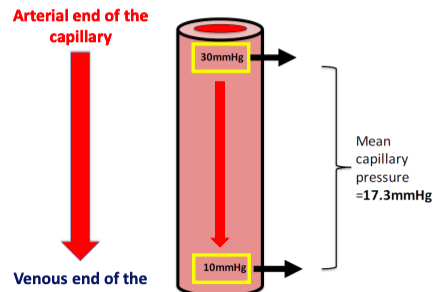
Net Filtration Pressure - Arterial end of capillary
Net Filtration Pressure - Venous end of capillary
The amount of filtration at each end is determined by the NFP and the permeability (Kf)
Notice the difference in pressure/amount of flow between the two ends of the capillary
~90% of the fluid leaving the capillary at the arterial end comes back into the venous end.
Venous end of capillaries have a higher Kf Filtration (mL/min) = Kf x NFP
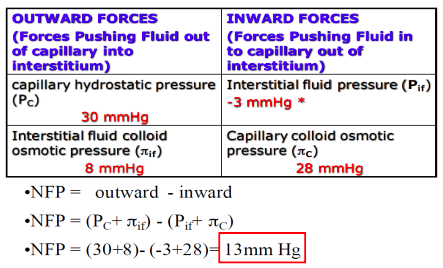
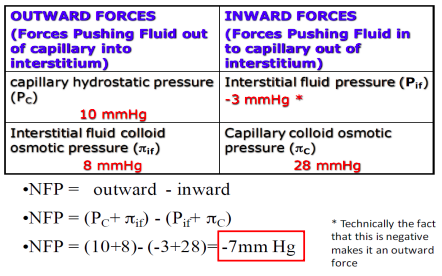
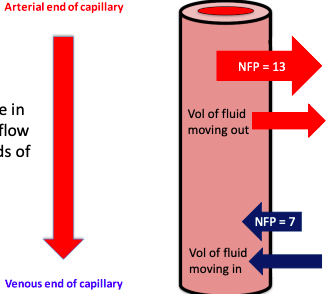
If 90% of the fluid comes back into the capillary, where does the remaining 10% go?
● To quantify: 10% = ~2ml/min
● Over the course of 24 hours = ~3000mL or 3L
● If this fluid was not removed, you would lose a lot of blood volume very quickly.
● The excess fluid left in the interstitial space (~3000mL/day) is drained away by the
lymphatic system.
Functions of the vena cava and veins
- ● When capillaries join together, they form post-capillary venules, which also serve as
exchange vessels, particularly for large macromolecules as well as fluid.
- ● An important feature of the venules and veins is that they contain valves at regular
intervals - this means blood flow in the venous system is unidirectional.
- ● As post-capillary venules join together and form larger venules, smooth muscle once
again appears.
- ● These venous vessels, like the resistance vessels, are capable of dilating and
constricting, and serve an important function regulating capillary pressure.
- ● Venules form larger veins that serve as the primary capacitance vessels of the body
- ie. the site where most of the blood volume is found and where regional blood volume is regulated.
○ For example, constriction of the veins decreases venous volume and increases venous pressure, which alters cardiac output by increasing venous return (preload).
● The final venous vessels are the inferior and superiour vena cava, which carry the blood back to the right atrium of the heart.
Arterial and venous compliance and capacitance
- ● The pressure-volume relationship (ie. compliance) for an artery and vein are
depicted:
- ● Two important characteristics stand out in the diagram:
- ○ First, the slope, which represents the compliance at a given pressure,
decreases as pressure increases.
- ○ Therefore, compliance decreases at higher pressures and volumes (ie.
vessels become “stiffer” at higher pressures and volumes).
- ○ Second, at lower pressures (venous pressure is usually less than 15mmHg),
the compliance of a vein is about 10 to 20 times greater than an artery.
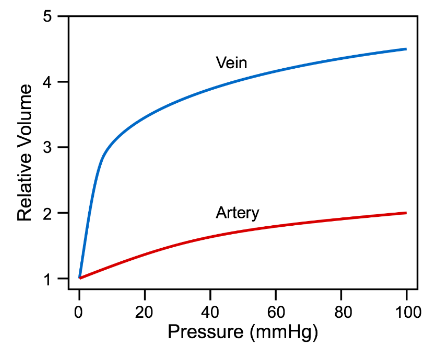
- ○ Therefore, veins can accommodate a large change in blood volume with only a small change in venous pressure.
- ○ At higher pressures and volumes, venous compliance is similar to arterial compliance (ie. the curve becomes flatter).
- ○ However, unlike the aorta and other large arteries, veins do not have a recoil response when stretched, thus they end up holding a greater amount of blood volume until the SNS tells them to contract.
- ○ For this reason, veins have a high capacitance and hold by far the largest volume of blood (~65%) within the system circulation.
Exercise, venous return and the skeletal muscle venous pump
- ● A major mechanism promoting high venous return during normal muscle contraction
is the muscle venous pump.
- ● Peripheral veins, particularly in the legs and arms, have one-way valves that direct
flow away from the limb and back toward the heart.
- ● Veins physically located within the large muscle groups undergo compression as the
muscles surrounding them contract, and they become decompressed as the muscles
relax.
- ● Therefore, with normal cycles of contraction and relaxation, the veins go through
stages of compression and decompression (ie. there is pumping).
- ● Muscle contraction propels blood forward through the open distal valve.
- ● During muscle relaxation, the proximal valves open and blood flows into and fills the
venous segment.
- ● The net effect is that the cycle of compression and relaxation propels the blood
towards the heart.
- ● Venous valves prevent the blood from flowing backwards, allowing only unidirection
flow that enhances venous return.
- ● When a person is standing, postural muscles in the legs alternately contract and
relax to keep the body in balance.
- ● This muscle actively promotes venous return and helps to maintain central venous
pressure and venous return, and lowers venous and capillary pressures in the feet and lower limbs.
The lymphatic system and vessels
- ● The lymphatic circulation runs in parallel to the systemic and pulmonary circulations.
- ● Flow in the lymph system begins in the tiny lymph vessels (think lymph capillaries)
that reside near the normal capillary bed in all tissues within the body.
- ● These vessels have lots of pores within their walls allowing the free movement of
fluid/protein/bacteria etc. into the lymphatic vessel.
- ● The lymphatic vessels rely on small pressure differences to provide flow of lymph
from tissues to the larger lymph vessels and ultimately back into the systemic circulation.
- ● The flow of lymph is unidirectional thanks to the pressure of valves (same as veins).
- ● Multiple lymph capillaries merge together and empty their contents into lymph nodes
- specialised structures that also contain a large number of lymphocytes and
macrophages that monitor the lymph for pathogens.
- ● The lymphatic system plays a critical role in the body to ensure that blood volume is
not lost in the interstitial space within tissues.
- ● Added up over the course of a day, the lymph collects ~3 litres of volume from the
interstitial space and returns this to the systemic circulation at the subclavian vein.
- ● Without the lymphatic system, more volume would remain in the interstitial space causing oedema/swelling and a depletion of blood volume over the course of the
day.
Regulation of lymphatic flow rate
What controls movement of fluid into lymph vessels?
- ● The amount of fluid delivered at capillary IS EQUAL TO the amount of fluid removed by lymphatics.
- ● If the volume of fluid at the capillary increases, accumulation of fluid in the interstitial space increases pressure.
- ● This leads to an increase in lymph flow rate to achieve a new steady state.
What happens if we exceed this max lymph flow rate?
- ● If the amount of fluid filtered a the capillary increases and exceeds max lymph flow
capacity:
●
- ● We get OEDEMA - swelling
What factors can cause oedema?
- High Kf = increased capillary permeability (eg. inflammation).
- High Pc = increased capillary hydrostatic pressure (eg. venous obstruction, volume
overload).
- High πif = increased interstitial protein content (eg. inflammation).
- Low πc = decreased capillary/blood protein (eg. liver disease).
- Low lymph flow = lymph blockage (eg. tumor, lymph node removal).
![]()
![]()
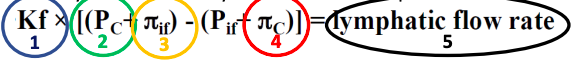
WEEK 11
Renal System 1 - Basic Renal Functions and Transport Processes in the Nephron Intro
- ● The urinary system consists of all organs involved in the formation, storage and release of urine.
- ● Includes the kidneys, ureters, bladder and urethra.
- ● Main function of urinary system is the eliminate the waste products of metabolism
from the body by forming and excreting urine.
- ● Usually between 1-2L of urine produced a day.
- ● Maintains homeostasis and enables excretion of waste from the body.
- ● This is a vital function and if renal function begins to decline, there are major and
deadly consequences for the rest of the body.
- ● What do the kidneys do?
- ● Excretory Functions (exocrine):
- ○ Excretion of metabolic waste and exogenous substances.
- ○ Body water balance
- ○ Electrolyte balance
- ○ Blood pressure regulation
- ○ Acid base balance
- ● Synthetic functions (endocrine):
- ○ Release of renin (activates renin angiotensin system)
- ○ Regulation of erythropoiesis
- ○ Synthesis of vitamin D
- ○ Gluconeogenesis (making glucose).
Excretory functions of the kidneys
- ● 10 million nephrons per kidney and come in 2 types:
- ○ Cortical nephrons (85%; excretory regulation)
- ○ Juxtamedullary nephrons (15%; concentrating/diluting urine).
- ● Despite there being 2 types, every nephron has the same
discrete sections:
- ○ Bowman’s (glomerular) capsule
- ○ Proximal convoluted tubule (PCT)
- ○ Loop of henle (LoH)
- ○ Distal convoluted tubule (DCT)
- ○ DCTs from a number of nephrons form together at
the distal end to form a collecting tubule that eventually turns into a collecting duct and delivers urine to the calyxes and eventually the ureters.
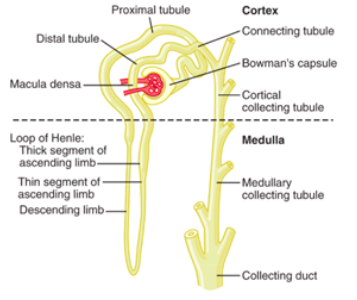
Events that determine renal excretion
- Filtration - the amount of plasma that is filtered from the blood
into the Bowman’s capsule from the glomerulus.
- Reabsorption - the amount of fluid/solute that is reabsorbed from
the nephron tubules back into the body.
- Secretion - the amount of fluid/solute that is secreted from the
peritubular capillaries that surround the nephron into the tubular network.
● Therefore the amount of any substance that is excreted from the body is determined using the following equation:
○ Excretion = Amount filtrated - Amount reabsorbed + amount secreted
Filtration
● The process where blood is filtered from the glomerular capillaries into the
Bowman’s Capsule through a serious of pores that make up the filtration barrier.
●
- ○ Afferent = incoming blood
- ○ Efferent = outgoing blood
- ○ Glomerulus = ball of capillaries
Glomerular filtration barrier Anatomy of the barrier:
- ● Fenestrated endothelium of glomerular capillaries
- ● Visceral membrane of the glomerular capsule made of podocytes and their
extensions (pedicles).
- ● THe intervening basement membrane composed of the fused basal laminas of other
layers.
Glomerular filtration barrier: size
- ● Filtration is a passive process
- ● Fluids and solutes are forced through the filtration barrier by hydrostatic pressure.
- ● Membrane allows molecules smaller than 3nm to pass freely out of the blood.
- ● The filtration therefore is essentially free of proteins (which are typically 7-9nm in
size).
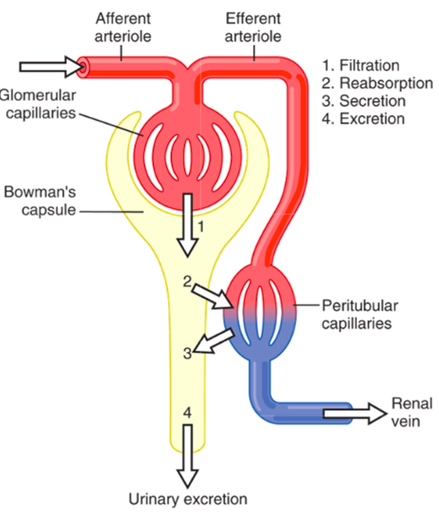
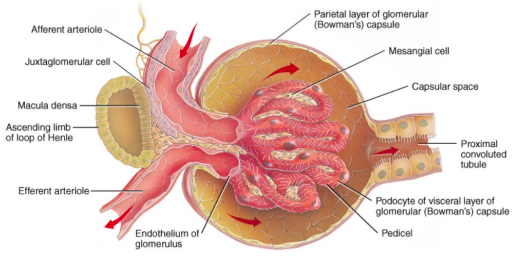
Glomerular filtration barrier: charge
- ● Capillary endothelial cells and podocytes have a negative charge
- ● This negative charge repels other negatively charged molecules (eg. proteins) and
prevents their passage into the filtrate.
○ In disease states (eg. glomerular nephritis or diabetic nephropathy) this
barrier lsoses its selectivity allowing protein into urine -> proteinuria. Glomerular filtration
- ● Filtrate formed
- ● ‘Ultra-filtrate’ of plasma
- ● Similar concentration of water, ions, small molecules (amino acids, glucose, etc) in
filtrate as plasma.
- ● Cells, platelets, and most plasma proteins do not enter Bowman’s capsule
Glomerular filtration rate (GFR)
- ● The kidneys receive about 20% of the total cardiac output.
- ● If resting CO is 5L/min, this means kidneys receive ~1L/min at any given time.
- ● Main function = make urine by filtering blood.
- ● Don’t filter blood but the plasma component of blood.
- ● If the haematocrit was ~60%, then 1L/min of blood flow equates to ~600mL of
plasma entering the kidneys per minute - ie. renal plasma flow is ~600mL/min.
- ● The rate at which filtrate is formed by nephrons from this 600m: of plasma is called
the glomerular filtration rate (GFR).
- ● In a young, healthy adult, GFR = ~125ml/min.
- ● This means that ~20-25% of plasma that enters the kidney each minute is filtered by
nephrons.
- ● A GFR of 125ml/min equates to 180L of plasma filtered each day.
- ● Given plasma volume is ~3L in the average person, this means our entire plasma
volume is filtered 60 times per day.
○ This provides the kidneys an enormous capacity to regulate fluid and
electrolyte levels in the body to ensure homeostasis.
- ● Renal blood flow is assumed to be constant but in reality, renal blood flow is tighly
regulated to ensure there is not too much or too little getting to the nephron where
filtration happens.
- ● How much blood enters the glomerular capillaries depends on the diameter of the
afferent and efferent arterioles.
- ● The body has multiple inputs that can control the diameter of these arterioles to
regulate how much filtrate is formed by the kidneys at any given time.
Control of glomerular filtration rate (GFR)
● GFR is dependent on 2 factors:
1. The surface area and permeability of the filtration barrier
○ Expressed as the constant Kf
2. The Net Filtration Pressure (NFP)
○ THe balance of hydrostatic and osmotic pressures at work across the filtration
barrier. GFR and NFP
NFP = outward - inward
Modifying GFR through arterial constriction
What happens if both vessels constrict?
Summary of arteriolar control of GFR
● The majority of physiological control of GFR occurs through alteration of the
glomerular capillary hydrostatic pressure (PGC)
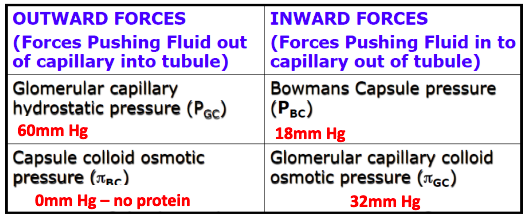
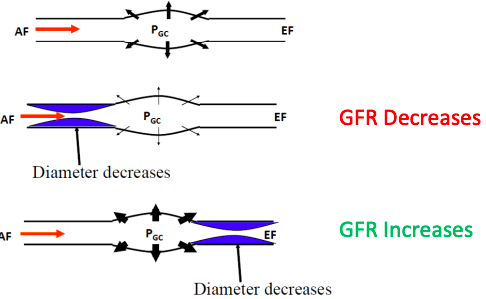
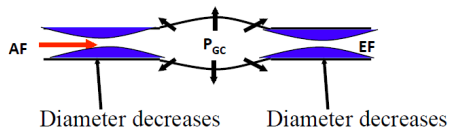
Renal Autoregulation
- ● Another physiological control mechanism exists within the renal arterioles that
ensure constant renal blood flow and GFR despite potential changes in systemic
blood pressure - this is autoregulation.
- ● Autoregulation is defined as the intrinsic ability of an organ to maintain a constant
blood flow despite changes in systemic blood pressure.
- ● This autoregulatory response occurs in the absence of neural and hormonal
influences and therefore is intrinsic to the organ, although these influences can
modify the response.
- ● Renal circulation manifests the phenomenon of autoregulation
- ● RBF remains relatively constant despite large changes in mean renal arterial
pressure.
○ Afferent and efferent arteriolar resistance are altered to keep GFR steady.
● Why is this useful?
○ Allows the kidneys to maintain their excretory capacity and blood volume
homeostasis even when systemic blood pressure changes. Renal Autoregulation: Myogenic mechanism
Vessel diameter unchanged, hydrostatic pressure increases, GFR increases.
Afferent arteriole constricts due to smooth muscle stretch-feedback, hydrostatic pressure and GFR largely unchanged.
Renal autoregulation: tubulo-glomerular mechanism
- ● As the Distal convoluted tubule wraps back around, it becomes
very close to the afferent and efferent arterioles.
- ● Macula densa within the DCL senses how much NaCl is coming
through the DCL.
- ● If the amount of NaCl decreases, it tells us that the flow of tubular
fluid has decreased.
- ● Macula Densa cells communicate with juxtaglomerular cells which
receive a signal which then causes them to produce more renin.
- ● This means increased angiotensin II which constricts efferent
arteriolar constriction and increases back pressure inside capillaries.
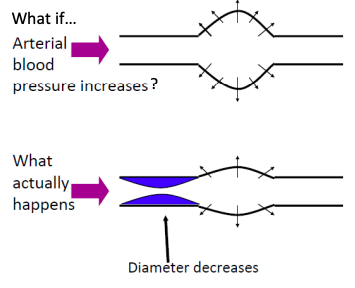
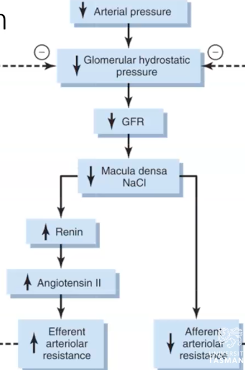
● Macula densa talks directly to afferent arterial to decrease resistance and increase dilation, increasing GHS.
Renal Autoregulation
● Myogenic mechanism
○ Very fast to act and accounts for ~50% of renal autoregulation. ● Tubulo-glomerular feedback mechanism
○ Slower to act and accounts for ~35-40% of renal autoregulation.
● Renal blood vessels can respond without SNS/humoral input to ensure a steady
GFR despite changes in systemic pressure.
Reabsorption and secretion
- ● Movement of fluid and substances from the tubular fluid back into the body is
through re-absorptive processes.
- ● Movement of solute from the blood into the tubules in the tubular network depends
on secretory processes.
- ● These are the only 2 processes available for water/solute movement for the tubular
network within the nephron (excluding bowman's capsule).
- ● Different solutes/substances are handled differently by the cells in the kidney but
most move between the tubule/capillaries by using either transcellular (through cells via transporters or ion channels) or paracellular (between cells using tight junctions) or both.
Principles of tubular handling of filtered substances
- ● Although reabsorption and secretion can take place across different parts of the
nephron, not every substance that is filtered can be reabsorbed and/or secreted.
- ● Waste products (eg. creatinine, organic anions and cations, drugs and metabolites).
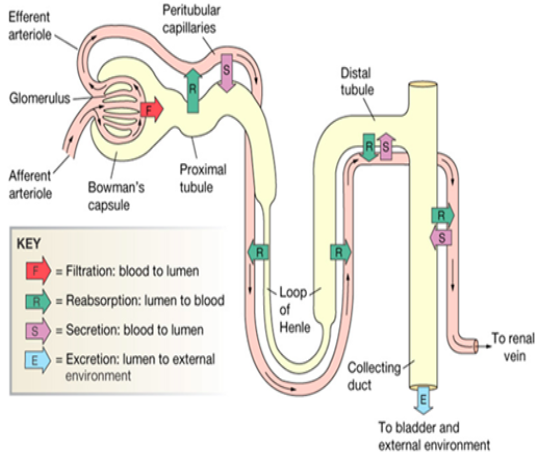
- ○ These are freely filtered at the glomerulus and there is no or very limited reabsorption.
- ○ The exception to this rule is urea.
- ○ There can be active tubular secretion to increase excretion rate or clearance
beyond what is filtered from the blood alone.
- ○ For this to occur, the nephron requires specific transporters to actively move
this substance from the peritubular capillaries and into the tubules.
- ● Nutrients (eg. glucose/amino acids)
- ○ These are freely filtered in glomerulus because they are small and not charged.
- ○ Evolutionarily, the body requires these nutrients to function normally.
- ○ Therefore, there is complete reabsorption (within limits) in the PCT of all
filtered nutrients.
- ○ There is no tubular secretion of these compounds.
- ● Sodium (Na+)
- ○ Na+ is key electrolyte in the body and has very high concentrations within th
blood (~145mmol/L).
- ○ Na+ is freely secreted at the glomerulus and given the large plasma
concentration, very large amounts of Na+ are filtered all the time.
- ○ There is variable reabsorption across the nephron that provides exquisite
control of how much Na+ is excreted from the body (depends on homeostatic
needs),
- ○ There is NO active secretion of Na+ in the nephron.
- ○ If Na+ retention is required: kidney can increase reabsorption of filtered Na+.
- ○ If Na+ excretion is required: kidney can reduce the amount of Na+
reabsorbed from the filtrate (allowing more to leave the body).
- ○ Na+ homeostasis is highly related to systemic blood pressure and the body
has multiple physiological inputs that can regulate Na+ balance.
- ● Potassium (K+)
- ○ K+ is a key electrolyte in the body and has relatively low concentrations within the plasma (~4.5mmol/L).
- ○ The low has concentration in the plasma means less absolute amount of K+ if filtered compared with other ions.
- ○ Variable reabsorption occurs across the nephron, giving exquisite control over K+ excretion.
- ○ If K+ retention is required: kidney can increase reabsorption of filtered K+.
- ○ If K+ excretion is required: kidney can reduce the amount K+ reabsorbed
from the filtrate. We can also switch on the active tubular secretion if further
K+ excretion is required.
- ○ K+ homeostasis is highly related to function of the heart, brain and muscles in
the body and the body has multiple physiological inputs that can regulate K+ balance.
● Water (H2O)
- ○ Large absolute amounts of water are filtered (180L per day) and forms 1-2 L
of urine per day.
- ○ There is near complete reabsorption of water in the nephron, the last few
percent (1-2L) can be reabsorbed or not depending on water homeostasis
requirements.
- ○ If H2O retention is required: kidneys can reabsorb nearly all filtered H2O.
- ○ If H2O loss is required: kidneys can simply fail to reabsorb some of the
filtered H2O.
- ○ Water homeostasis is highly related to systemic blood pressure and the body
has multiple physiological inputs that can regulate water balance.
The limits of tubular transport
- ● The majority of the ‘work; that takes place in the nephron is reabsorption.
- ● This is because the absolute amount of plasma filtered each day at the glomerulus
far exceeds our total blood volume (by a factor of 30) and thus most of what is
filtered must be reabsorbed back into the body to maintain homeostasis.
- ● When we consider how much reabsorption can actually take place in the nephron,
there are two limiting factors to be aware of:
- The rate of reabsorption of certain substance (eg. glucose) is limited by the absolute
number of transporters available that can move that substance from the tubular
lumen back into the body. Therefore reabsorption can be ‘Transport Limited’
- The rate of reabsorption of certain substances (eg. electrolytes) is limited by the
maximum concentration gradient that can be achieved and maintained between the tubular lumen and the interstitial space. Therefore reabsorption can be ‘Gradient Limited’
Transport limits and osmotic diuresis
Reabsorption of some substances is rate-limited by transporter capacity
- ● Nutrients and organic wastes have an upper limit to the rate at which they can be reabsorbed from the tubular lumen back into the body.
- ● This upper limit is called the tubular maximum-limited (Tm) system (aka it’s the transporter max).
Glucose transport in the nephron
- ● Glucose is freely filtered in glomerulus
- ● Passive co-transport occurs only in the proximal tubule in conjunction with Na+
- ● Passive transport into peritular capillaries down concentration gradient
○ Note - blood flow prevents glucose build up in interstitial space, so conc. Gradient is maintained to favour glucose movement into the bloodstream.
Glucose is a good example of Tm limited transport
● At normal glucose concentrations (~5mM) all of the filtered glucose is reabsorbed.
- ● When glucose levels rise and filtered load = Tm then glucose begins to appears in the urine.
- ● Note that secretion/reabsorption of glucose by kidneys does not normally regulate plasma [glucose]
○ Although some SGLT2 inhibitors (reduce Tm) are used to reduce glucose levels in people with T2D.
Gradient limited transport - eg. Na+ in the proximal tubule
● ●
●
Some substances (typically ions) do not have a Tm
Upper limits of transport is defined by the concentration gradient that can be sustained across the tubule.
Eg. as Na+ is reabsorbed in the proximal tubule,
○ Lumen [Na+] decreases while interstitial [Na+] increases
○ The tight junctions in the proximal tubules are slightly leaky to Na+
○ The gradient limit for Na+ is only 2 mmol/L, once the interstitial [Na+] exceeds
tubular lumen [Na+] by more than 2mmol/L, Na+ starts to leak back into the
tubules through paracellular pathways (tight junctions become leaky).
○ Under normal circumstances in the proximal tubule, water follows Na+ so that
the lumen [Na+] doesn’t change and is not anywhere near the gradient limit.
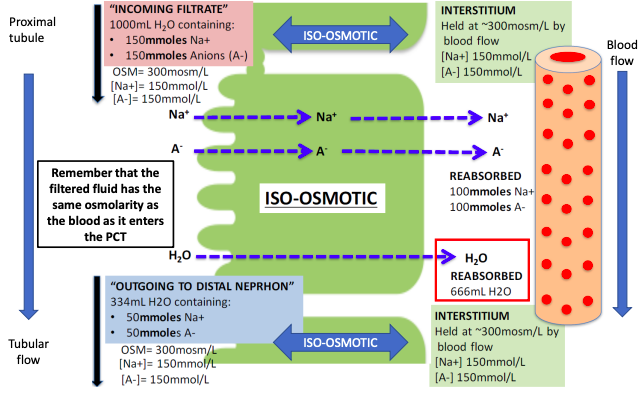
This is ●
●
the normal situation... what happens if we stuff things up with an ‘osmotic diuretic’ What if we intravenously infuse a patient with a solute that stays in the extracellular fluid, is freely filtered but poorly reabsorbed in the kidney.
Eg. Mannitol
- ○ If we infuse enough mannitol to increase extracellular fluid osmolarity to 400 mosm/L
- ○ Of course, the glomerular filtrate derives from the extracellular fluid so it will also increase to 400 mosm/L...
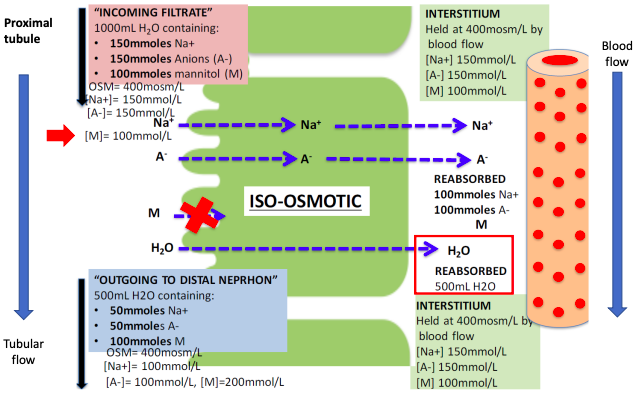
Osmotic diuresis - mannitol/water
- ● Before mannitol
○ 1000ml fluid into tubule from glomerulus
○ 334ml fluid out of distal nephron as urine (65% reabsorbed)
- ● After mannitol
○ 1000ml fluid filtered into tubule from glomerulus.
○ 500ml fluid out of distal nephron as urine (50% reabsorbed)
- ● Therefore, mannitol causes an osmotic diuresis
Osmotic diuresis - mannitol and Na+
- ● Before mannitol
○ [Na] in interstitium and peritubular capillaries is 150 mmol/L
○ [Na] in tubular fluid going out of distal nephron as urine is 150 mmol/L
- ● After mannitol
○ [Na] in interstitium and peritubular capillaries is 150 mmol/L
○ [Na] in tubular fluid going out of distal nephron as urine is 100 mmol/L
- ● Therefore, mannitol (and the water that goes with it) has diluted the tubular [Na+]
- ● But remember that Na+ reabsorption is gradient limited
Late PCT with Osmotic Diuretic
Osmotic diuresis
- ● The result of osmotic diuretics such as mannitol is:
- ○ Increased water delivery to the distal nephron - “diuresis”
- ○ Increased Na+ delivery to the distal nephron - “natriuresis”
- ● These overwhelm the absorptive capacity of the distal nephron and lead to
increased volume of urine, quickly causing dehydration.
- ● Mannitol is not the only osmotic diuretic
- ● If blood glucose levels exceed the Tm of glucose, then the excess glucose cannot be
reabsorbed out of the lumen.
- ● Glucose increases the osmotic pressure in the lumen and, like mannitol, it drags
water and Na+ out with it to increase urine output.
○ This is why uncontrolled diabetics urinate a lot and are always thirsty.
Take home message: there are limits on tubular transport
- ● Renal transport can be limited by the Tm (eg. glucose)
Or
- ● Renal transport can be gradient limited (eg. Na+)
- ● Osmotic diuresis is a good example that demonstrates what happens when we reach
these transport limits.
WEEK 12 - Renal System 2 - Water, Sodium and Potassium homeostasis
Variation in water and solute handling across the nephron
- ● Different parts of the nephron perform different tasks
- ● This is largely dependent on the relative differences in reabsorptive and secretory
processes available to each segment.
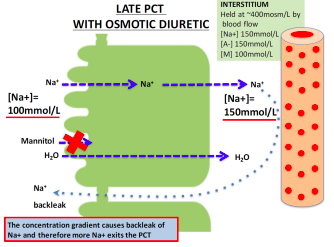
● Most of the physiological control of water, Na+ and K+ occurs in the distal nephron and collecting duct.
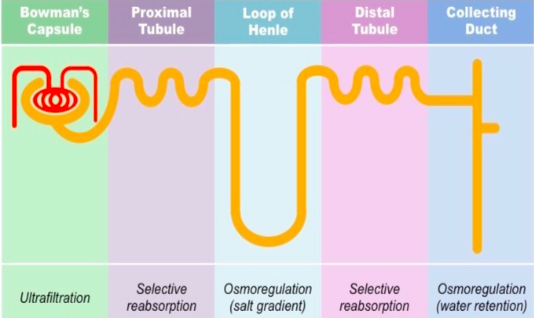
●
Antidiuretic Hormone (ADH)
- ● ADH is released from the posterior pituitary gland in response to dehydration or low
blood volume.
- ● ADH travels through the blood stream to the kidney where it interacts with receptors
on principal cells to increase water reabsorption in the distal nephron (inserts
aquaporin 2 channels into cells).
- ● Normally the distal nephron is impermeable to water but in the presence of ADH it
becomes permeable.
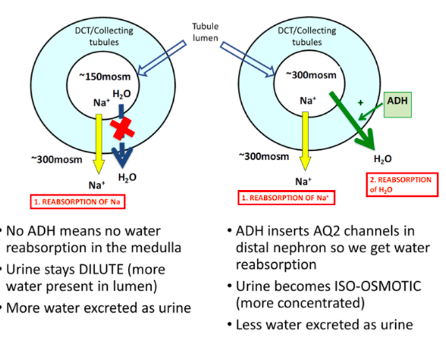
- ● When ADH is present, the distal nephron can increase water reabsorption to concentrate the tubular fluid (and hence urine) to ~300mosm/L.
- ● However, the kidney is able to further concentrate the tubular fluid to a maximum of ~1200mosm/L.
- ● This occurs thanks to the organisation of the nephron and the blood vessels that surround the tubules in the deeper parts of the renal medulla - called ‘counter current mechanism’.
Counter-current mechanism and renal interstitial solute concentration
- ● The renal medulla, the inner part of the kidney, consists of the medullary collecting
ducts, loops of henle, vasa recta (capillaries) and the interstitial space.
- ● The unique spacial arrangement of these components is essential for the regulation
of urine concentration and other specialised kidney functions.
- ● The extent to which we can concentrate our urine is dependent on two factors:
- ○ 1. The presence of ADH
- ○ 2. The interstitial concentration of solutes in the medulla.
Video 1
The role of the counter-current mechanism and ADH in urine formation
What mechanism produces the concentration gradient in the renal medulla?
● There are 2 main factors:
- ○ 1. Flow of tubular fluid in opposite directions (aka the counter-current multiplier) in combination with:
- Variation in water permeability across the loop of henle
- Active Na+ transport in the thick ascending limb of the look of henle.
- ○ 2. Slow blood flow through deep medullary capillaries (vasa recta) allows the
concentration of interstitium to increase.
1. Loop of Henle permeability to water and solute
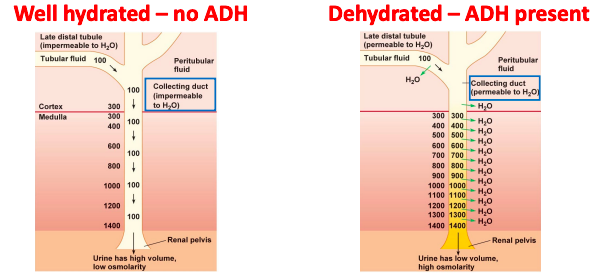
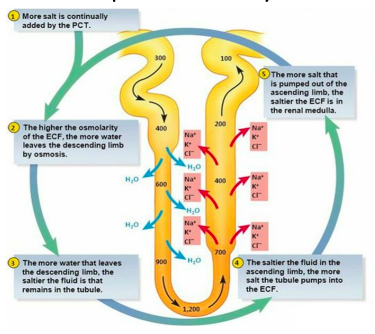
2. Medullary blood flow
○ 1. Medullary blood flow is much slower than in the cortex
■ While useful for concentrating urine, this does increase the susceptibility of the medulla to ischemic injury.
○ 2. The arrangement of the medullary blood supply into the descending and ascending vasa recta limits the washout of the medulla hyperosmolarity.
Countercurrent Mechanism Countercurrent exchange
- ● Process in which the exchange of solutes and water takes place passively between descending and ascending limbs of vasa recta.
- ● Vasa recta acts as a countercurrent exchanger.
- ● H2O is removed from descending limb of LOH into the hyperosmolar interstitium,
which is taken back by ascending limb of vasa recta.
- ● Thus, vasa recta prevents dilution of osmolality of interstitium.
- ● Solutes come out of ascending limb of LOH -> ensures trapping of solutes in the
medulla.
- ● Thus, the exchange of water and solutes between descending and ascending limbs
of vasa recta maintains the osmolality gradient established by LOH.
Urea and the osmolarity in the medulla
- ● NaCl is not the only player
- ● Only half (~600 mosm/L) of the maximum possible osmolarity (~1200 mosm/L) is
from NaCl.
- ● The remainder (~600 mosm/L) is due to UREA
Recycling of urea in the distal nephron
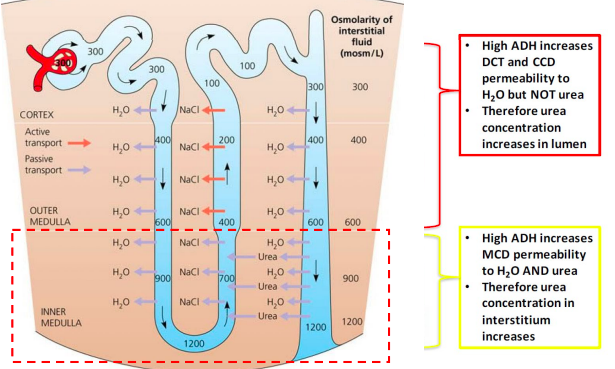
How much urine volume do you need to produce each day for excretory homeostasis?
- ● The amount of solute excreted daily varies on diet
- ● Generally, for a 70kg person, a minimum amount of solute of 600mOsmoles must be
excreted by the kidney daily.
- ● This contains Na, K, Cl, urea, creatinine, urate, phosphate, etc.
- ● This 600mOsmoles can be excreted in as little as 500ml (max ADH effect) of water
per day or as much as 15L (min ADH effect) per day.
- ● This 500ml is called the obligatory urine volume and is set by the maximum
concentration of the urine that can be achieved in the renal medulla according to the following equation:
○
- ● This minimal loss of volume in the urine contributes to dehydration, aong with water
loss from the skin, respiratory tract, and GIT, when water is not available to drink.
- ● The limited ability of the human kidney to concetrate the urine to a maximal
concentration of 1200 mOsm/L explains why severe dehydration occurs if one
attempts to drink seawater. Can you drink seawater to survive?
- ● NaCl concetration in the oceans averages about 3.0-3.5%, with an osmolarity ~1200mOsm/L.
- ● Drinking 1 L of seawater wit a concetration of 1200mOsm/L would provide a total NaCl intake of 1200mOsmoles.
- ● If maximal urine concentrating ability is 1200mOsm/L, the amount of urine volume needed to excrete 1200 milliosmoles would be 1200 milliosmoles divided by 1200mOsm/L or 1.0L.
- ● Why then does it cause dehydration?
- ○ The kidney must also excrete other solutes/wastes, especially urea, which
contributes about 600mOsm/L.
- ○ Thus, for every L of seawater drunk, 2L of urine would be required to rid the
body of 1200 milliosmoles of NaCl ingested in addition to all the other soltes.
- ○ Therefore, don’t drink seawater bc it will accelerate body water loss!
Water homeostasis
- ● Approximately 50-60% of our body weight comes from water.
- ● Within the body, water is located in 3 compartments:
- ○ 1. The intracellular space (~28L of body wt)
- ○ 2. The interstitial space between cells (~10L body wt)
- ○ 3. In the blood vessels as plasma (~3.5L body wt)
- ○ (these are all averages of a 70kg human)
- ● A variety of pathological conditions induce abnormalitites in fluid balance.
- ● Fluid abnormalitites are either an overload of fluid or a decrease in effective fluid.
- ● Fluid overload is clinically known as oedema.
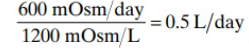
- ● Oedema occurs most commonly in soft tissues of the extremities; however it can occur in any tissue.
- ● Decreases in fluid are commonly referred to as dehydration and this can manifest as altered cardiovascular function due to low filling pressure (ie. insufficient volume to fill the blood vessels).
- ● Therefore, the body has a number of defense mechanisms that aim to maintain body water homeostasis and prevents both fluid overload and dehydration.
- ● The kidneys play a major in regulating how much water leaves the body and the thirst response regulates how much water we drink each day.
Video 2
Physiological Regulation of body water Basic negative feedback loops in physiology
● These mechanisms have two components:
- A sensor that detects a change in either the physical or chemical property that has a
specific set-point (eg. vascular stretch).
- The sensor sends a signal to an effector that mediates a change opposite to the
original disturbance (tries to restore homeostasis).
Body water homeostasis
- Body ○ ○
- Body ○ ○
water sensors are:
Osmoreceptors in the hypothalamus Baroreceptors (mechano-receptor)
water effectors are:
Posterior pituitary release of ADH (signal from hypothalamus)
ADH increases water permeability in the distal nephron (and increased inner medullar urea absorption).
Activation of the thirst centre increases thirst (and thus water intake)
○
Blood volume, baroreceptors and ADH release
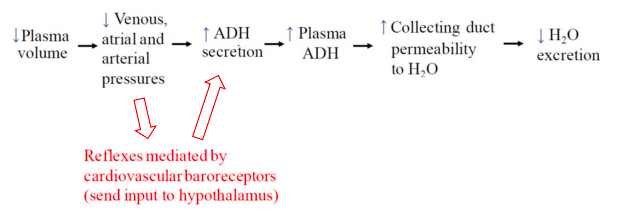
Interplay of the osmoreceptor, thirst and ADH
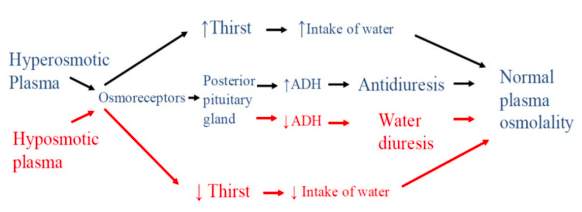
Which is the more powerful mediator of ADH release?
Secretion of ADH
● ADH secretion depends on both plasma osmolarity and volume: 1. Plasma osmolarity
○ Very sensitive: responds to 1-2% change 2. Plasma volume
- ○ Less sensitive: responds to 10-15% change
- ○ But higher maximum ADH release beyond 15%
- ● In many instances increased plasma osmolarity and decreased blood volume go
together and become synergistic (eg. dehydration, blood loss with fluid shift from
extracellular space).
- ● Sometimes these sensors are in opposition (eg dehydration with excessive
hypotonic fluid replacement; eg. diarrhoea with normal water intake) and the result
will be determined largely by how low blood volume is.
- ● In a tug of war, marked hypovolaemia (baroreceptor input) will win
Water homeostasis and thirst
- ● Water balance is achieved by balancing input (via thirst) with output (formation of
dilate or concentrated urine via ADH).
- ● ADH levels and thirst are stimulated by high plasma osmolarity to regulate water
homeostasis...
- ● Disorders of water balance often manifest as disorders of plasma Na+ concentration.
Sodium Homeostasis
- ● In sodium-deficient states, salt consumption is driven by salt appetite - an innate and
motivated behavioural response that drives us to seek and ingest salt-containing
foods and fluids.
- ● However, currently, the ambient salt in the diet is in excess of physiological need.
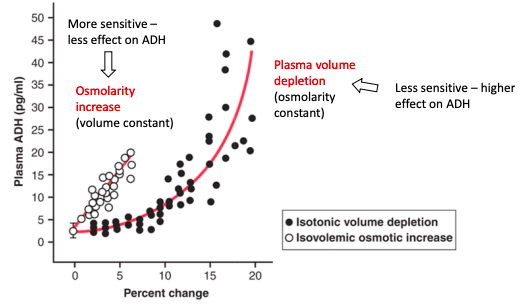
- ● Clinical trials provide definitive evident of the direct cause-and-effect relationship between salt consumption and high BP.
- ● Moreover, increased salt intake in the long term has been linked with not just blood pressure increases but also blood vessel dysfunction, heart failure and kidney failure.
- ● Thus, despite being critical for our normal bodily functions, too much salt has significant consequences and causes disease.
- ● Luckily, we have physiological control over salt content in the body and our kidneys work hard to maintain salt homeostasis by regulating how much salt we excrete in urine at any given time.
Why is salt so important?
- ● Sodium (Na+) is the dominant cation present in the extracellular fluid (ECF) and has
a concentration of ~145 mmol/L.
- ● Because Na+ has such a high ECF concentration, Na+ and its accompanying anion,
chloride (Cl-) make up most of the plasma osmolarity (~300mOsm/L) under normal
physiological conditions.
- ● Therefore, controlling the ECF Na+ concentration is almost the same as controlling
plasma osmolarity.
- ● Regulation of plasma Na+ concentration is achieved by regulating input of water
(thirst) and output of water (kidneys, ADH status) and mediated by the osmoreceptors.
- ○ When we have a low Na+ concentration in the ECF we call this hyponatraemia
- ○ When we have a high Na+ concentration in the ECF we call this hypernatraemia
- ● Water follows Na+ in the ECF
- ● Where there is a high concentration of Na+, there is usually lots of water as well.
- ● Therefore when we have a high amount of Na+ in the ECF, this leads to a higher
ECF volume.
- ● In the cardiovascular system, this means a higher vascular filling volume and
therefore increased BP.
- ● Therefore controlling the amount of Na+ in the plasma is the same as controlling
ECF volume (and thus BP)... because plasma osmolarity is monitored closely by the
osmoreceptors, water balance is adjusted to maintain a constant 300mOsm/L.
- ● When we have a high amount of Na+ in the body, we have high ECF volume and
therefore low blood pressure because osmolarity is held constant.
- ● Summary: controlling the amount of Na+ in the body is the same as controlling blood
volume and therefore blood pressure.
- ● The physiological control of Na+ in the body involves the kidneys, the renin
angiotensin aldosterone system and the atrial natriuretic peptide system.
Video 3
Control of Na+ in the body by the RAAS and ANP Renal regulation of Na+ excretion
● As Na+ is freely filtered at the glomerulus and actively reabsorbed, but not secreted then:
- ○ Sodium excretion = Na+ filtered - Na+ reabsorbed
- ○ = (GFR x PNa+) - Na+ reabsorbed Physiological regulation of Na+
Negative feedback looks for Na+:
1. A sensor ->
○ 1. Extra-renal pressure sensors
■ Baroreceptors (activate CNS)
○ 2. Intrarenal pressure sensors
■ Juxtaglomerular cells (release renin)
○ 3. Indirect pressure sensor (NaCl delivery)
■ Macula desna (renal autoregulation/GFR control)
2. An effector ->
- ○ 1. A change in the GFR
■ AND/OR
- ○ 2. A change in Na+ reabsorption
Regulation of Na+ by increased GFR
● Increasing arterial pressure causes a corresponding rise
in filtrate formation and therefore renal water output. ○ This is called pressure diuresis
● Increasing arterial pressure also causes a corresponding increase in Na+ filtration and therefore output.
○ This is called pressure natriuresis
● Therefore if pressure goes up, Na+ excretion goes up.
Regulation of Na+ by reduced GFR
Regulation of Na+ by reabsorption
● There are a number of stimuli that increase tubular reabsorption of Na+ (and
therefore Na+ in the body).
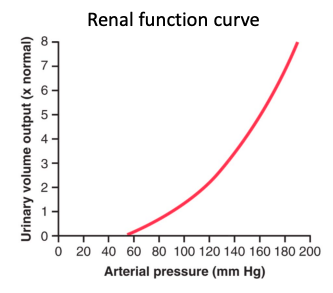
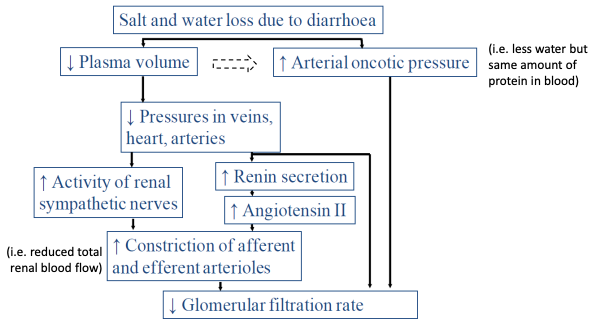
- Increased angiotensin II
- Increased aldosterone
- Increased sympathetic tone and catecholamines
- Increased filtration fraction (altering starling’s forces in peritubular capillaries to
increase reabsorptive processes).
- Decreased atrial natriuretic peptide (ANP)
Renin angiotensin system in Na+ (and BP) homeostasis
Effect of angiotensin II on Na+ and water handling
- Angiotensin stimulates the Na+/H+ antiporter in the proximal convoluted tubule
causing Na+ reabsorption utilising H+ cycle
- Angiotensin II also stimulates the Na+/K+ ATPase to move Na+ into the interstitial
space at the expense of K+.
- ○ Since water follows Na+, Angiotensin II increases Na+ and water
reabsorption in the proximal nephron..
- ○ Angiotensin II also stimulates release of aldosterone in the adrenal gland
Effect of aldosterone on Na+ and water handling
- Aldosterone increases the number of Na+ channels in the apical membrane.
- Aldosterone increases Na+ transport into the interstitial space by activating Na/K
ATPase
● Remember that Na+ and water can be handled independently in the distal nephron
so while aldosterone will increase Na+ reabsorption, water will follow only if ADH is
also present.
What is we need to increase Na+ excretion?
- ● Many cells in the atria of the heart can secrete ANP
- ● The main stimulus for ANP secretion is distention of the atria
- ● ANP acts directly to inhibit Na+ reabsorption in the distal nephron (collecting duct)
- ● ANP also inhibits the RAAS
○ Results in less water and Na+ reabsorption. What happens when we increase total body sodium?
(eg high salt diet)
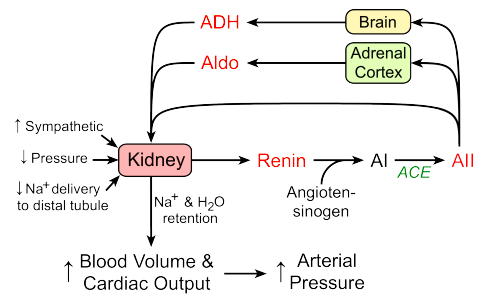
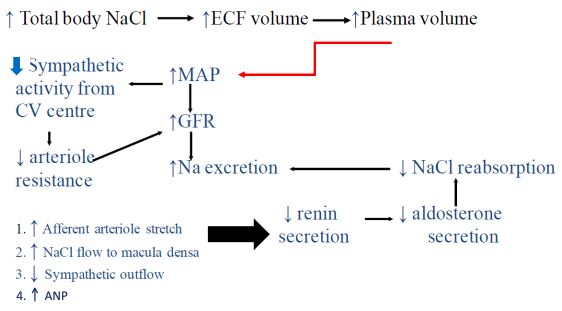
Summary
- ● The amount of Na+ in the body determines the ECF volume and therefore plasma
volume
- ● Our body works hard to control how much Na+ is in the body because this
determines blood pressure.
Potassium Homeostasis
- ● There is very little K+ in the ECF (~4mmol/L).
- ● Despite having low concentrations in the ECF, K+ exists at very high concentrations
(~140mmol/L) within cells.
- ● Thus, K+ is the dominant cation within cells and it is fundamental to
depolarisation/repolarisation in excitable cells like neurons and cardiomyocytes.
- ○ 98% of the K+ in the body resides in cells and 2% resides in the ECF
- ○ The body works very hard to ensure ECF concentration of K+ is held between
3.5-5mmol/L.
- ○ This very narrow range is achieved by tightly controlling how much K+ is
excreted from the body via the renal system.
How do we regulate potassium homeostasis?
- ● Over the course of the day we take in approximately 100 mmol of K+ from our diet.
- ● If we assumed that the ECF volume is ~14L in total (plasma + space between cells)
then adding 100 mmol of K+ would increase the ECF K+ concentration by more than 3.5mmol/L.
○ We would almost double our plasma K+ concentration from ~4-7.5mmol/L if nothing else happened.
- ● Luckily, our body has a rapid defence mechanism built in that prevents the dietary K+ from increasing our ECF K+ concentration.
- ● This defence mechanism involves multiple hormones and their ability to stimulate the movement of K+ into cells when plasma K+ levels increase.
- ● In doing so, the dietary K+ goes into a compartment in the body (cells) where the K+ concentration is already really really high (~140 mmol/L).
- ● Therefore, adding a little K+ from the ECF to each cell does not greatly change the intracellular concentration of K+.
- ● This movement of K+ into cells as the ECF concentration goes up is a very fast homeostatic mechanism that buffers and potential change in the overall ECF K+ concentration.
- ● However, we cannot sustain this indefinitely and we need another way of regulating the amount of total K+ in the body -
○ this is where the renal system comes in and helps regulate the output of K+ to match the input from the diet.
●
Video 4
Physiological control of body K+ occurs in the distal nephron
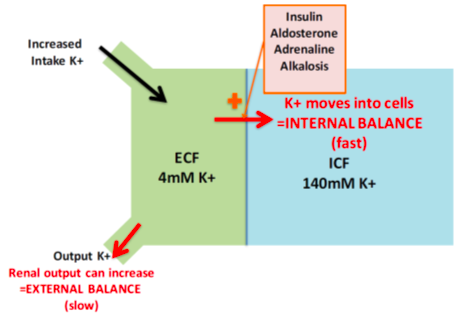
How ●
How ●
● How ●
● How ●
●
is K+ excretion regulated
As with other solutes, K+ is freely filtered, actively reabsorbed and variably secreted:
- ○ Potassium excretion = K(filtered) - K(absorbed) + K(secreted)
- ○ = (GFR x Pk) - K(reabsorbed) + K(secreted) K+ is reabsorbed in the PCT
Reabsorption of K+ in the PCT is constant whether it's hypokalemia or hyperkalemia.
○ Ie. 65% of filtered load is reabsorbed
Reabsorption of K+ is passive through both paracellular and intracellular routes. K+ os reabsorbed in the ascending loop of Henle
Thick ascending limb
- ○ Transport mechanisms are both passive and active
- ○ Active transport from the interstitium into cells entourages further passive
movement back into interstitium
Loop diuretics act here to directly cause hypokalemia
is K+ reabsorbed in the distal nephron?
Intercalated cells can reabsorb some K+ in exchange for H+
When we need to conserve K+, the processes in the distal nephron shuts down K+ secretion by principal cells, leading net reabsorption by intercalated cells
Three main factors influence K+ secretion by principal cells in the distal nephron: 1. Aldosterone status
- Plasma K+ concentration
- Na+ and water deliver to the distal nephron (ie. luminal flow rate in the tubule)
Effect of aldosterone
- ● Renin release stimulates aldosterone release
- ● If aldosterone high, increased K+ secretion
Effect of plasma K+ concentration
- ● Direct effect on principal cells
- ● Increased uptake via basolateral pump due to increased substrate - increasing
secretion.
Effect of tubular flow rate on K+
- ● The relative K+ concentration difference between intracellular and lumen of distal nephron is influenced by the flow rate of the luminal fluid.
- ● Luminal fluid is related to our hydration status and the total volume of urine being formed (ie. during diuresis we have fast tubular flow).
Does water status affect K+ homeostasis?
● Less ADH: decreased collecting duct permeability to ions
● More ADH: increased collecting duct permeability to ions.
Effect of high Na+ diet on K+ balance
- ● High Na+ diet leads to reduced aldosterone which leads to decreased K+ secretion
in principal cells.
- ● However, Na+ also increases GFR and decreases PCT reabsorption therefore
increasing K+ secretion.
- ● These effects counterbalance each other and there is little effect on K+ excretion.
Summary of potassium homeostasis
- ● Regulation of renal K+ excretion rate is by changing the rate of distal K+
reabsorption and especially secretion.
- ● Regulators of K+ secretion in the principal cells are:
○ Aldosterone
- ○ Plasma K+ concentration
- ○ Luminal flow rate in the distal nephron.
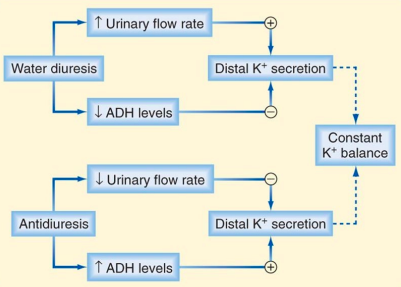
Summary of Renal Physiology 2
- ● The kidneys are the main regulators of water and electrolyte balance in the body.
- ● Proper homeostatic regulation of Na+ is critical for maintenance of blood volume and
pressure because water will follow Na+ in the body.
- ● When the amount of Na+ in the body changes, the body can activate a number of
systems to normalise Na+ and these work largely by activating thirst or changing
renal handling of Na+ via RAAS or ANP.
- ● Homeostatic control of ECF K+ concentration is critical to ensure our excitable cells
are able to depolarise and repolarise.
- ● When K+ concentrations in the ECF changes, this can lead to rapid and serious
consequences and so K+ concentrations in the ECF are closely monitored and maintained at low concentrations by modulating the renal excretion rate of K+.
Week 13: Acid Base Physiology
Reintroduction - Acids and Bases
- ● pH is a measure of the H+ concentration in a solution (eg the ECF in the body)
- ● It is calculated as pH = -log[H+}
- ● We can rearrange this to solve for H+: [H+] = 10-pH
- ● Eg. normal pH = 7.4 then [H+] = 40 nmol/L
- ● As the [H+] goes down then pH goes up (alkaline) and vise versa
- ● 40 nmol/L is not very much and therefore a small increase or decrease in H+ then
pH goes up or down quite quickly.
- ● When H+ is lost from the body, pH increases quite quickly if nothing else happens
- ● Therefore there are powerful physiological mechanisms that help regulate the
concentration of H+ in the ECF to ensure constant pH.
- ● Why does pH need to stay constant:
- ○ Because all enzymes and chemical reactions are set up to work at a pH of ~7.35-7.45.
- ○ If you go beyond this range, the body very quickly and dramatically start to dysfunction (above 7.8) or shut down (6.8).
- ● 3 main mechanisms:
- ○ The buffer system
- ○ Respiratory Control
- ○ Renal compensation
Video 1: reintroduction to Acids and Bases
● Acid: substance that is capable of releasing a proton.
○ hydrogen/proton DONOR
○ HCL->H++Cl-
● Base: substance that can accept/take a proton
- ○ hydrogen/proton ACCEPTOR
- ○ HCO3- + H+ -> H2CO3
Strong vs Weak Acids and Bases
- ● A strong acid or base fully dissociates in solution
○ HClH++Cl-
- ● Weak acid or base partly dissociates
○ H2CO3 H+ + HCO3 - Equilibrium Constant (Ka)
- ● This constant describes how much of the acid dissociates in solution
- ● Is calculates as:
- ● The larger the Ka = stronger acid
○ Counter-intuitive with pH change
- ● ThepKaisthe-logoftheKa
- ● Smaller pKa = stronger acid
●
Control of pH in the body
- ● If H+ production was not balanced by H+ removal, we would quickly reach a fatal acidic pH (pH ~6.8)
- ● Fortunately extracellular pH is kept at pH 7.4 (7.35-7.45)
○ Within cells pH is a little more acidic (~7.2) due to production of H+.
● Control of [H+] or pH in the body is a result of the balance between:
- ○ 1. Processes that produce H+
■ AND
- ○ 2. Processes that decrease H+
Acid production in the body
- ● We produce ~1300 mmoles of H+ a day
- ○ Fixed acids (~70 mmoles/day)
- From the food we eat
- Metabolism of proteins and fats
- Removal by kidneys
- ○ Volatile acids from cellular metabolism -CO2
- ~1000 - 2000 mmoles/day
- Removal by the lungs
- ○ Fixed acids (~70 mmoles/day)
- ● How does CO2 effect H+ levels?
○
Processes that decrease plasma [H+]
● Three levels of defence: 1. Buffer Systems
○ Works incredibly quick (fraction of a second) 2. Respiratory Control
○ Eg. removal of volatile acids by breathing out
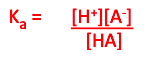
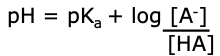
![]()
○ Works in minutes 3. Renal Compensation
- ○ Excretion or retention of HCO3-
- ○ Hours to days
Buffer Systems in Acid-Base Homeostasis
- ● Buffers are our first line of defence against pH changes in cells, ECF and urine.
- ● Buffers work almost instantaneously by binding and excess H+ produced and
forming a new molecule.
- ● If conditions allow it, the molecule that accepted the H+ can also release the H+
back into solution if the amount of H+ starts to decrease.
- ● This means the H+ binding reaction is reversible and depends on the concentration
of H+ and the buffer in the solution.
- ● The primary job of buffers is to stall any pH change that might occur so that the other
systems (respiratory or kidney) can kick in and remove the excess H+. Video 2: A Theoretical Experiment - Buffering H+
The bicarbonate buffer system is the most important buffer in the body
- ● This buffer system involves bicarbonate and carbonic acid
- ○ Bicarbonate = HCO3- = conjugate base
- ○ Carbonic acid = H2CO3 = weak acid
- ● This buffer system works by binding H+ using bicarbonate (there is lots of
bicarbonate in the plasma and interstitial fluid) to momentarily form carbonic acid that then quickly breaks down into water and CO2 according to:
○
- ● The two sides of the equation are in equilibrium
- ● If one side increases then the other side does too
- ● The equation below explains how the bicarbonate buffer system regulates acid-base
physiology:
○
- ○ [HCO3-] stands for the plasma concentration of bicarbonate and pCO2 is the
partial pressure of CO2 in the expressed air.
- ○ KEY MESSAGE: HCO3- : pCO2 determines pH
- ● If ratio goes up then the ratio goes up and thus pH goes up & vise versa.
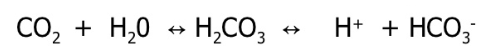
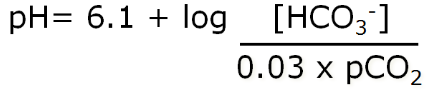
● We have physiological control over the pCO2 (lungs) and the HCO3- (kidneys).
The Respiratory System in Acid-Base Homeostasis
- ● The respiratory system contributes to the balance of acids and bases in the body by
regulating the blood levels of carbonic acid (H2CO3).
- ● CO2 in the blood readily reacts with water to H2CO3, and the levels of CO2 and
H2CO3 in the blood are in equilibrium.
- ● When the CO2 level in the blood rises, the excess CO2 reacts with water to form
additional H2CO3, which dissociates further into H+ and HCO3- thus lowering blood
pH.
- ● Increasing the rate and/or depth of respiration allows you to exhale more CO2
- ● This helps push H+ and HCO3- back to H2CO3 and subsequently CO2 and water
- ● Therefore the lungs can work to regulate the ratio of HCO3- to CO2 by modulating
how much CO2 is removed from the system
●
- ● To do this, pCO2 is closely monitored by chemoreceptors and even a tiny change
activates the respiratory centre.
- ● When our ECF pH becomes acidic, increased respiration is stimulated.
- ● This occurs when CO2 is produced because the carbonic acid-bicarbonate transition
kicks in and the equation favours the right hand side more.
- ● When our EFC becomes more alkaline, respiration is inhibited... but only to a point
because of the need for O2 supply.
- ● This sort of physiological control of pH is possible when dealing with volatile acid, such as CO2 produced from oxidative phosphorylation.
- ● This is because the CO2 produced by cells diffuses out into the blood (& combines with water to make some H+), the blood washes it away and delivers it to the lungs.
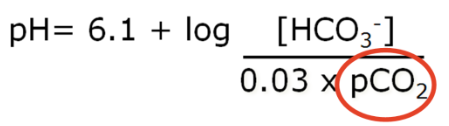
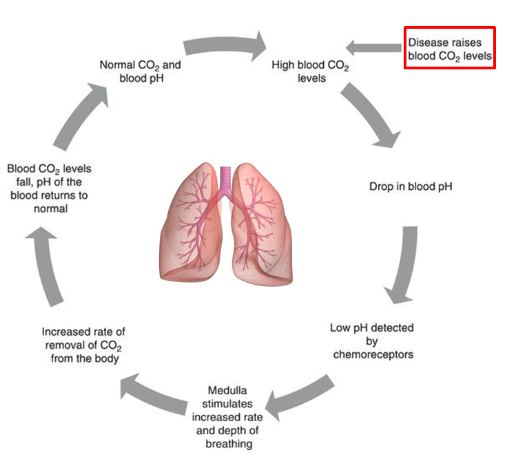
- ● In the lungs, H+ is converted back in CO2 and H2O and the CO2 is removed from the body.
- ● In this way we are not using up bicarbonate
- ● In contrast, when we produce fixed acids, we end up using bicarbonate to buffer the
H+ being released and thus form more CO2 and H2O.
- ● The CO2 can be removed from the system to help deal with excess H+ production
thus preventing a pH change.
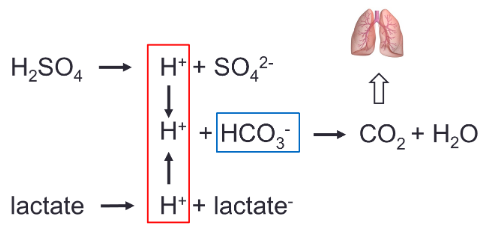
●
- ○ In thi example: for every 1 H+ added, we lose 1 HCO3- and gain 1 CO2
- ○ The new CO2 is exhaled but we are 1 HCO3- short compared to where we
started.
- ○ The result of this is a loss of HCO3- in the plasma.
- ○ If nothing else were to happen, our HCO3- levels would quickly be depleted
and we would not be able to regulate pH.
The Renal System in Acid-Base Homeostasis
- ● The buffer and respiratory systems are essential for dealing with fast changes in pH -
they act quickly.
- ● In contrast the kidneys have multiple mechanisms that enable them to regulate the
concentration of HCO3- and H+ in the body.
- ● For this reason, the kidneys are the most powerful, long-term regulators of pH in the
body.
- ● The kidneys work really hard to regulate the amount of HCO3- in the body and in
doing so, they regulate the ratio of HCO3- to CO2 and thus pH:
●
- ● The normal concentration of HCO3- in the body is ~24 mmol/L
- ● Like all other ions in the body, HCO3- is freely filtered in the nephron and across the
course of a day, ~4320 mmoles of HCO3- is filtered.
- ● If we didn’t have mass reabsorption of HCO3- taking place in the kidney, it wouldn’t
take long to deplete our body HCO3- levels.
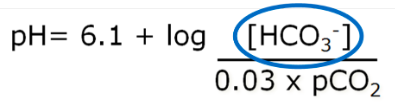
● The nephron can increase or decrease reabsorption of HCO3- to suit the body’s needs by changing what is happening in the PCT, DCT and collecting ducts.
VIDEO 3: Regulation of HCO3- and H+ in the Kidneys
- ● Bicarbonate reabsorption: PCT 85%
- ● Renal HCO3- handling:
○
- ● Synthesis of new HCO3-
- ○ Reabsorption of filtered HCO3- just stops us from losing what we already have
- ○ To excrete fixed acids we need to make new HCO3-
- ○ Renal tubular cells can make HCO3- from CO2 and H2O and get rid of the
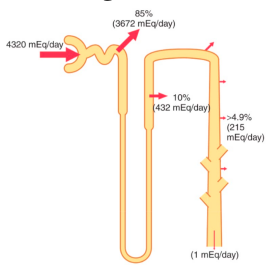
H+ formed in the process
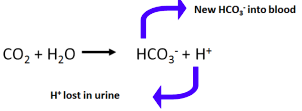
○ ● How
○ The answer is to bind the H+ (buffer it). ● Urinary Buffers and H+ excretion
- ○ H+ bound to urinary buffers is called titratable acidity
- ○ HPO42- + H+ <-> H2PO4- is the major buffer in urine
- ○ Up to 40 mmoles of H+ can be bound up in this way before urinary buffers are
much H+ can you excrete in urine?
- ○ Minimum urine pH = 4.5
- ○ pH 4.5 = only 0.03mM free H+
- ○ We need to get rid of ~70 mmoles of fixed acids per day.
- ○ Would need to make 2000L of urine/day
used up.
much H+ can you excrete in urine?
● How
- ○ 70 moles fixed acid produced a day
- ○ Therefore, 70 mmoles new HCO3- must be made to buffer excess H+
production.
○ Therefore, the 70 moles of H+ made in the process must be
secreted/excreted (because we make HCO3- by secreting H+).
- ● Take home message:
○ Addition of fixed acid is really loss of bicarbonate.
○ Excretion of fixed acid is really gain of new bicarbonate. - ● Amino acid biochemistry (basic version)
○ ○
Breakdown of amino acids (protein metabolism) produces NH4+ Ammonia is toxic and converted to either urea which is excreted by the kidneys or glutamine which is converted back to NH4+ in PCT.
- ● PCT
- ○ 1. PCT cells metabolise glutamine to NH4+ and HCO3-
- ○ 2a. This weak acid NH4+ is secreted into the filtrate, taking the place of H+
on a Na+ - H+ antiport carrier.
- ○ 2b. For each NH4+ secreted, a bicarbonate ion enters the peritubular
capillary blood via a symport carrier.
- ○ 3. The NH4+ is excreted in the urine.
- ● Regulation of ammonia excretion
- ○ Decreased extracellular pH leads to:
- Increased hepatic glutamine production (therefore less NH4+ converted to urea, more NH4+ converted to glutamine).
- Increased glutamine breakdown to HCO3- and NH4+ in PCT
- ○ NH4+ excretion can increase to ~300 mmol/day, but it takes several days to
- ○ Decreased extracellular pH leads to:
achieve this maximum.
- ● Summary: Tubular H+ secretion results in:
○ 1. Reabsorption of filtered HCO3- (PCT_
■ Once HCO3- is fully reabsorbed, any further tubular H+ secretion
results in:
○ 2. Synthesis of new HCO3- (DCT/CD). Secreted H+ binds to urinary buffers.
■ Once urinary buffers are used up:
○ 3. Further H+ secretion and HCO3- generation occurs via ammonia (NH4+)
secretion/excretion (PCT). ● 2nd take home message:
- ○ Net gain or loss of HCO3- due to renal acid-base regulation depends on the balance of 3 processes:
- ○ 1. The amount of titratable acidity excreted ○+
○ 2. The amount of NH4+ excreted ○-
○ 3. The amount of HCO3- lost in the urine.
Acid Base Pathophysiology and Compensation to maintain pH Definitions:
- ● Acidaemia = decreased blood pH
- ● Acidosis = A pathological process that decreased pH
- glutamine handling
- ● Alkalaemia = increased blood pH
- ● Alkalosis = A pathological process that increases pH
- ● You can have pathology leading to both a metabolic alkalosis which tends to raise pH and a respiratory acidosis that tends to lower pH.
- ● In this scenario, you can have metabolic and respiratory disturbances leading to no apparent alkalemia or acidaemia, but despite the lack of pH change the pathology is still present.
The 4 pathological processes that can disturb pH:
1. Metabolic Acidosis:
- ● The issue with metabolic acidosis is always loss of HCO3- from the body.
- ● This can be due to direct losses from the gastrointestinal tract or through increased
buffering of H+.
- ● Loss of HCO3-
●
- ● Case: diabetic Ketoacidosis (DKA)
- ○ Cant use glucose so increase in fixed acid prouction in the form of ketone bodies from fatty acid metabolism.
- ○ Acetoacetic acid -> acetolacetate- + H+
- ○ Beta-hydroxybutyric aicd -> Beta-hydroxybutyrate.
- ● H+ buffered by HCO3-
- ● Respiration increases to remove CO2
- ● Kidneys being secreting H+, making new HCO3-, forming titratable acidity and
excretion ammonia.
- ● Compensation - metabolic acidosis
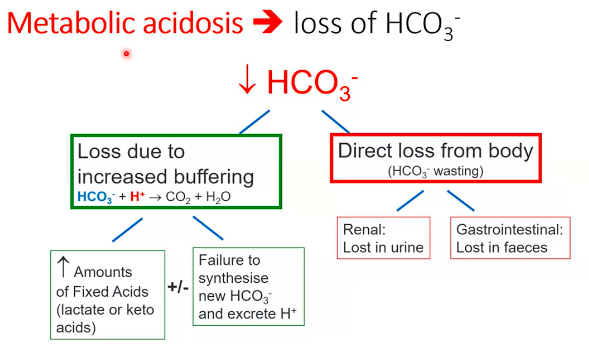
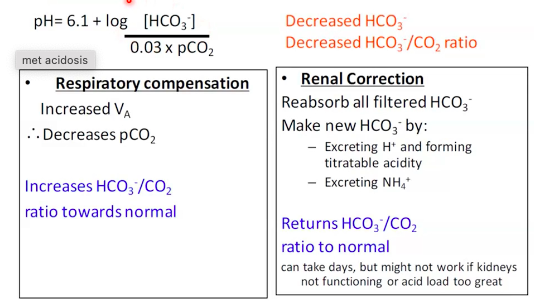
●
2. Metabolic Alkalosis
- ● The issue with metabolic alkalosis is always a gain of HCO3- in the body.
- ● This can be due to direct losses of H+ from the body (vomiting) or inappropriate gain
of HCO3- (antacids/renal problems).
- ● Causes:
- ○ Loss of H+, gain of HCO3- (eg.vomiting)
- ○ Ingestion of base (antacids)
- ○ Inappropriate renal HCO3- conservation and production
■ ECF volume depletion
■ Hypokalaemia
■ Hyperaldosteronism (RAAS)
● Case: Excessive Vomiting
- ○ 22 yr old woman with 4 days of epigastric pain, vomiting, weight loss and
dehydration.
- ○ Net result: loss H+, gain of HCO3-
○
- ○ Respiratory system tries but is focusing on other things
- ○ Kidney does the main work
- ○ But the person is dehydrated so:
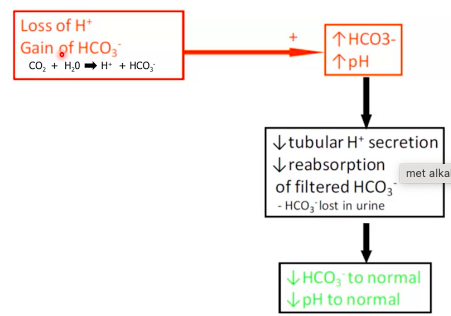

○
- ○ ECF depletion and HCO3- reabsorption:
- Increased Na+ to conserve ECF volume leads to increased H+ secretion
- Net result is increased HCO3- reabsorption into plasma - worsens alkalemia.
- ○ Hypokalaemia and synthesis of HCO3- DCT/CT - intercalated cells
- Alkalosis leads to a shift of K+ from ECF into cells
- Increasing K+ reabsorption leads to increased amount of H+ secretion
- For every H+ we secrete, we make a new HCO3- -worsens alkalemia
- ○ Aldosterone and synthesis of HCO3- DCT/CT - intercalated cells
- Aldosterone stimulates the H+/ATPase in intercalated cells leading to
increased H+ secretion.
- For every H+ we lose, we make a new HCO3- -worsens alkalemia.
- ● THerefore we are making the whole cycle worse:
●
- ● Respiratory compensation:
- ○ Decreased Va therefore increased pCO2
- ○ Decreases HCO3-/CO2 ratio towards normal
■ But limited by need to maintain oxygenation ● Renal correction:
- ○ Partially reabsorb filtered HCO3- ie. let some HCO3- be lost
- ○ Returns HCO3-/CO2 ratio to normal
■ But mechanism doesn’t work if volume depleted, decreased K+, aldosterone, etc.
3. Respiratory Acidosis
● The issue with respiratory acidosis is always a gain of CO2 in the body.
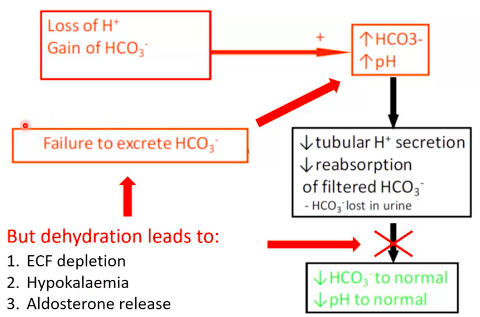
- ● This can be due to hypoventilation or a compromised respiratory system (eg, asthma).
- ● Caused primarily by hypoventilation leading to increased pCO2 ○ CO2+H2O->H++HCO3-
- ● Can be caused by:
- ○ Diseases of the airways (eg. asthma)
- ○ Obstructive sleep apnoea
- ○ Severe obesity (limits lung expansion)
- ● Essentially anything that limits normal inflation/deflation of the lungs and hence breathing.
- ● Respiratory compensation: ○ None
- ● Renal Compensation
- ○ Reabsorb all filtered HCO3-
- ○ Make new HCO3- by:
- Excreting H+ and forming titratable acidity
- Excreting NH4+
- ○ Increases HCO3-/CO2 ratio towards normal.
4. Respiratory Alkalosis
- ● The issue with respiratory alkalosis is always a loss of CO2 in the body. This can be
due to hyperventilation from a panic attack, stress/anxiety or certain drug use.
- ● Caused primarilty by hyperventilation leading to decreased pCO2
- ● CO2+H2O<-H++HCO3-
- ● Can be caused by:
- ○ Panic attack
- ○ stress/anxiety
- ○ Drug use
- ● These can manifest acutely and will have quick effects on acid-bas homeostasis
- ● Remeber that it takes the kidneys time to activate
- ● Breathing in paper bag means we are re-breathing our expired CO2 to try and stop
the alkalosis.
- ● Compensation:
- ● No respiratory compensation
- ● Renal compensation:
- ○ Partially reabsorb filtered C+HCO3- ie. let some HCO3- be lost
- ○ Decreases HCO3-/CO2 ratio towards normal
- ○ Takes time to activate
Summary
● Acid base disturbances are a major challenge in the body and manifest as a change
in the pCO2 to HCO3- ratio.
- ● Our defence mechanisms can work together to limit the acid base disturbance, thus preventing us from reaching a deadly pH.
- ● These defence mechanisms are the buffer systems, the respiratory system and the renal system and they work synergistically to regulate pH at any given time.
●