Cell Biology: Microbiology (Practical)
Investigating the effect of antiseptics on the growth of bacteria
In this experiment you will prepare a lawn plate of bacteria before testing the effectiveness of three different antiseptics. Care must be taken when handling microorganisms such as bacteria. You will use techniques called aseptic techniques during this experiment to avoid contamination. Contamination can be where microorganisms from:
The surroundings get into your experiment and spoil your results
Your experiment get into the surroundings and cause a potential health hazard.
You will measure the diameter of the ‘clear zone’ around the disc where there is no bacteria growing. The larger the clear zone, the more effective the antiseptic.
METHOD
A nutrient agar plate
A Bunsen burner
A heatproof mat
A disposable plastic pipette
A culture of bacteria (E. coli)
A glass spreader
Filter paper discs
Three antiseptics (such as mouthwash, TCP, and antiseptic cream)
Disinfectant bench spray
A ‘discard beaker’ of disinfectant
A small beaker of ethanol
Forceps
Clear tape
Hand wash
A wax pencil
Access to an incubator
1) Set up your working area by first spraying the bench with the disinfectant spray and wiping with paper towels.
2) Place the Bunsen burner on the heatproof mat in the middle of your working area and light the Bunsen on a yellow flame.
3) Wash your hands with the antibacterial hand wash.
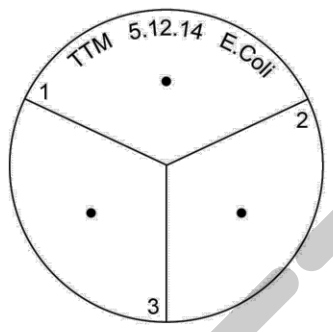
4) Mark the underneath of a nutrient agar plate (not the lid) with the wax pencil as follows (making sure that the lid stays in place to avoid contamination):
Divide the plate into three equal sections as if you were cutting a pie into three and number them 1, 2 and 3 around the edge
Place a dot into the middle of each section
Around the edge write your initials, the date and the name of the bacteria (E. coli).
5) Turn the Bunsen flame to blue.
6) Remove the lid of the bottle containing the culture of bacteria (keep the lid in your hand) and flame the neck of the bottle through the Bunsen flame, quickly twisting the bottle from side to side. Using the disposable pipette, collect approximately 1 ml of the bacterial culture.
7) Quickly flame the neck of the bottle again and replace the lid.
8) Lift the lid of the agar plate at an angle so that it is only fully open on the Bunsen burner side.
9) Pipette the bacteria onto the agar plate and replace the lid.
10) Place the pipette into the ‘discard beaker’ and turn the Bunsen burner flame back to yellow.
11) Dip the glass spreader into the ethanol. Remove the glass spreader and tap off the excess ethanol, then pass the glass spreader through the flame (holding the glass spreader horizontally to ensure nothing drips down onto your hand).
12) Allow the flame on the glass spreader to go out and allow the spreader to cool for a count of 20.
13) Lift the lid of the agar plate, again at an angle so only the side next to the Bunsen burner is fully open, and spread the bacteria around the plate using the glass spreader.
14) Lower the lid of the agar plate and place the glass spreader into the discard beaker.
15) Place different antiseptics onto the three filter paper discs by either soaking them in the liquid or spreading the cream or paste onto them.
16) Lift the lid of the agar plate as before and, using the forceps, carefully place each disc onto one of the dots drawn on with the wax pencil.
17) Make a note of which antiseptic is in each of the three numbered sections of the plate.
18) Secure the lid of the agar plate in place using two small pieces of clear tape (do not seal the lid all the way around as this creates anaerobic conditions, which will prevent the E. coli bacteria from growing and can encourage some other very nasty bacteria to grow).
19) Incubate the plate at 25 °C for 48 hours.
20) Measure the diameter of the clear zone around each disc by placing the ruler across the centre of the disc. Measure again at 90° to the first measurement so that the mean diameter can be calculated.
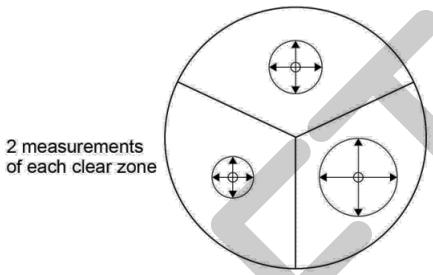
21) Record your results in a table.

60 Copyright © 2015 AQA and its licensors. All rights reserve
 Knowt
Knowt