Quantum Mechanics
Wavefuctions
Wavefunction (Ψ) - describes the movement of a particle
Probability density (Ψ2) - probability of finding the particle in a region
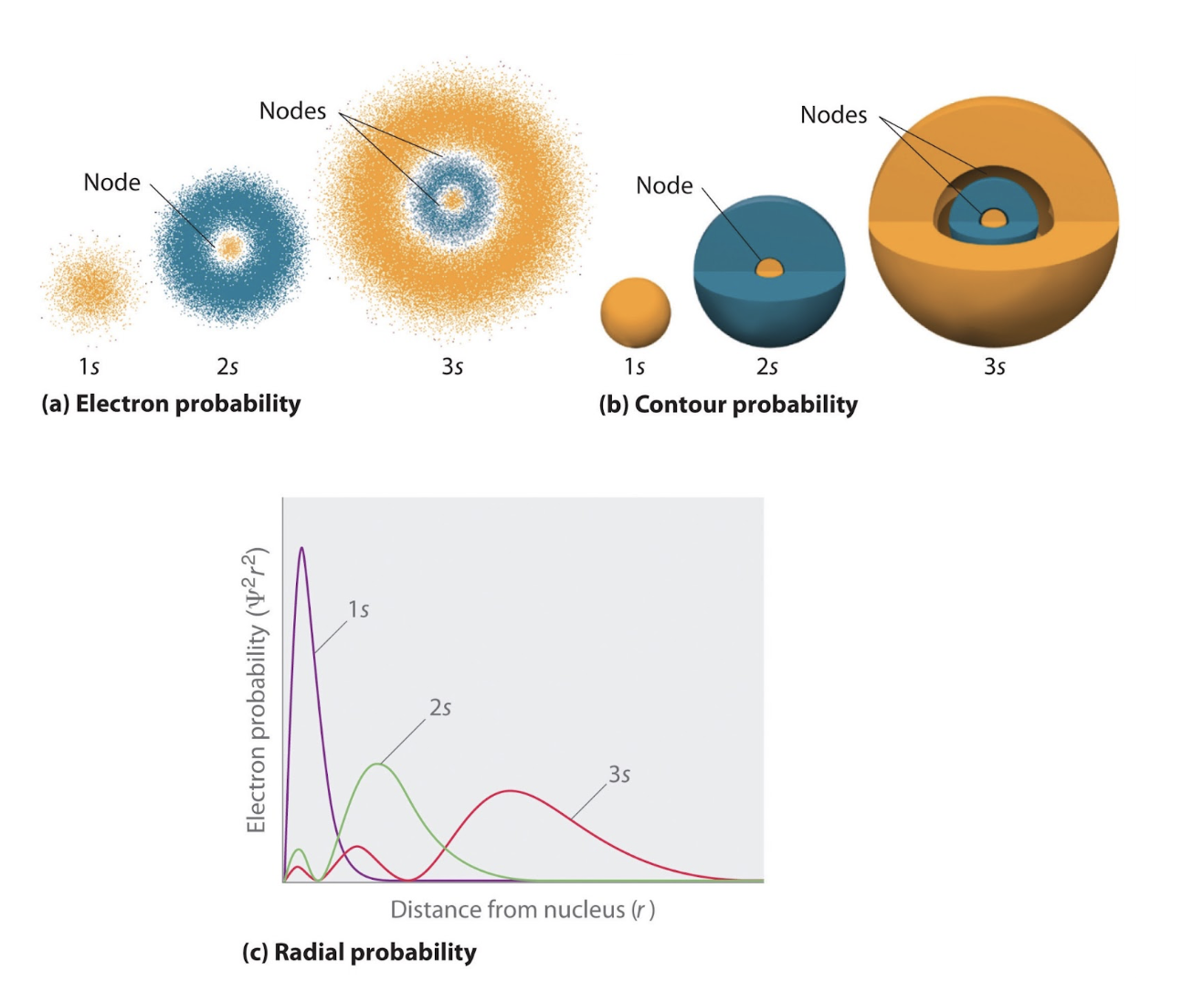
Node - where the probability of finding an electron is zero
Wavefunctions have (n-1) nodes
Nodes
Can occur whenever a wavefunction, and probability density, is 0
An electron can never be at a node
Can “cross” nodes, because they are delocalized/standing waves
S orbitals
Exists for all energy levels n ≥ 1
L = 0
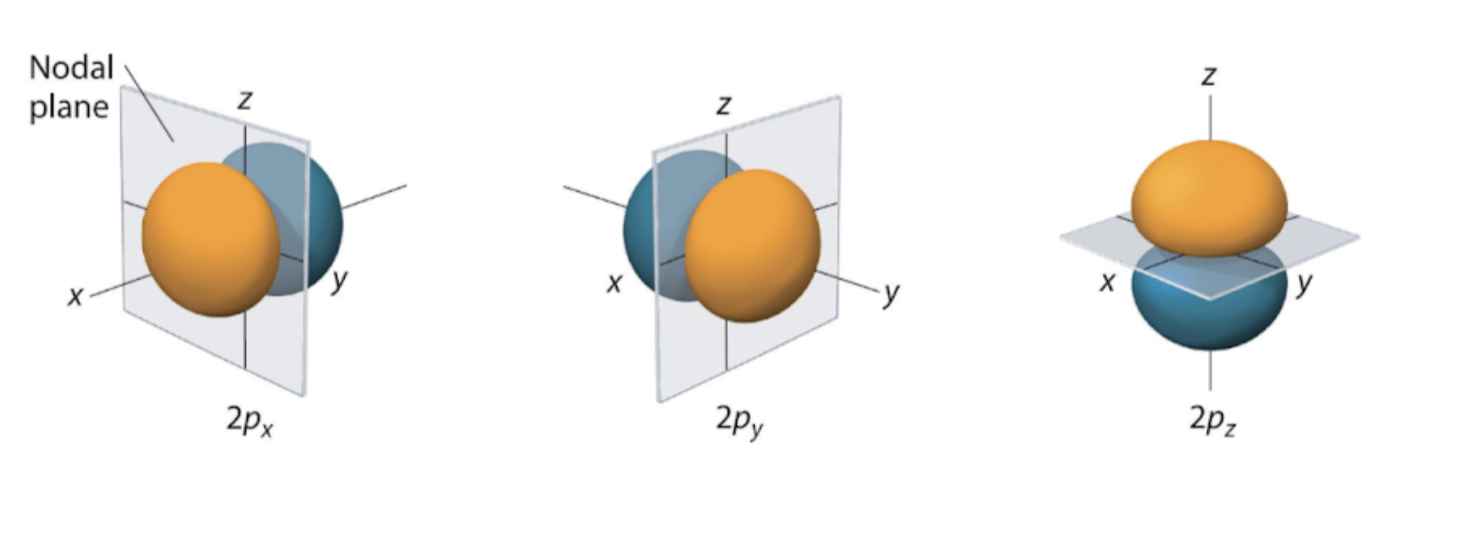
P orbitals
Exists for n ≥ 2
L = 1
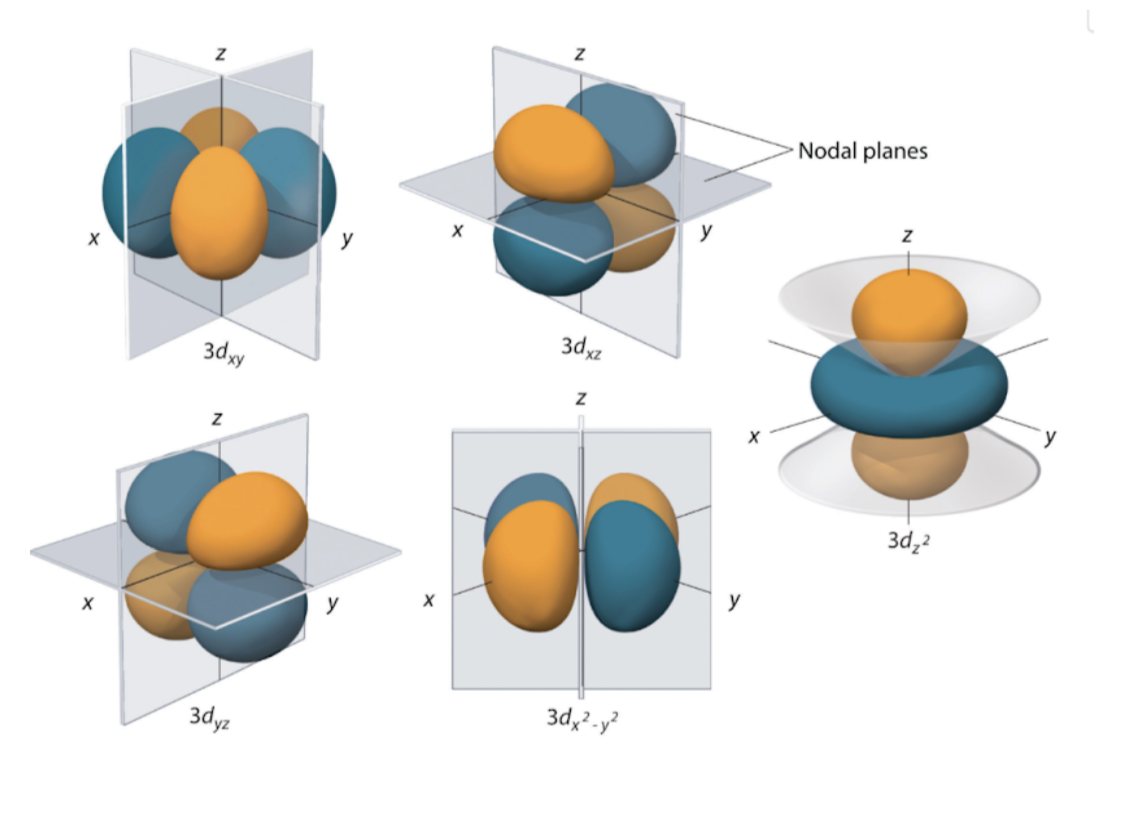
D orbitals
Exists for n ≥ 3
L = 2
Shielding and Penetration
Orbitals are a delocalized cloud of electron density
Penetrating - orbitals with radial probability closer to the nucleus
The closer, more penetrating orbitals are shielding the further orbitals from the nucleus
Diamagnetism vs Paramagnetism
Paramagnetic - has unpaired electrons
Drawn towards magnets
Diamagnetism - has paired electrons
Slightly repelled by magnets
Principle quantum number (n)
Principle quantum number - defines the size of and energy of the orbital
Must be positive whole numbers
Number of orbitals in a shell
n2
Angular momentum (l)
Angular momentum - defines the 3D shape of the orbital
Can be any integer from 0 to (n-1)
Number of different shapes of orbitals per shell
n
Magnetic quantum number (ml)
Magnetic quantum number - defines the spatial orientation of the orbital
Allowed values are from -l, …, 0,... +l
Number of spatial orientations of orbitals in a subshell
2l + 1
s sublevel = 2 electrons.
p sublevel = 6 electrons.
d sublevel = 10 electrons.
f sublevel = 14 electrons
Spin quantum numbers (ms)
Spin quantum number - defines spin axis of electron
Allowed values for each orbital are +½ or -½
Only two electrons allowed per orbital