Unit 9: The Mole and Stoichiometry
Measuring Matter
mole - the number of atoms of carbon in exactly 12 grams of carbon-12
Avogadro’s number, NA - 602,214,076,000,000,000,000,000 atoms of carbon; rounds to 6.022 × 1023
one mole of paper would make a stack that would reach to the moon more than 80 billion times
a mole of nickels stacked up would reach to the sun and back
one mole of inches would be 1,616,434 light years, or across our galaxy and back 8 times
one mole of seconds is about 19 quadrillion year, 4,240,66 times the age of the earth, or 954.150 times the age of the universe itself
one mole of pennies could repay the United States National Debt 86 million times
how we will use the mole:
first, we will need to be able to convert from moles to particles. when we say particles, the might mean atoms, molecules, ions, or formula units, whichever we are talking about, the math works the same
so, how many atoms are in 2.00 moles of iron?
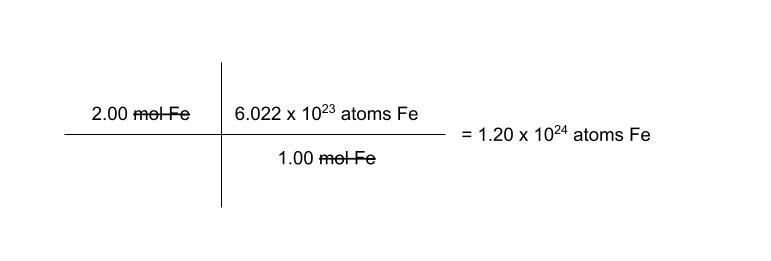 *remember to label every number very specifically. and yes, the abbreviation for mole is mol
*remember to label every number very specifically. and yes, the abbreviation for mole is mol
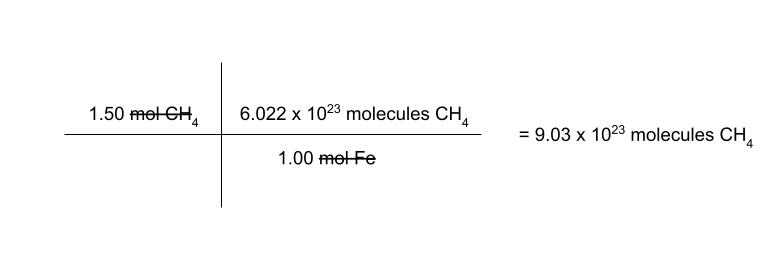
how many molecules are in 1.50 moles methane?
we also will have to be able to move from particles to moles:
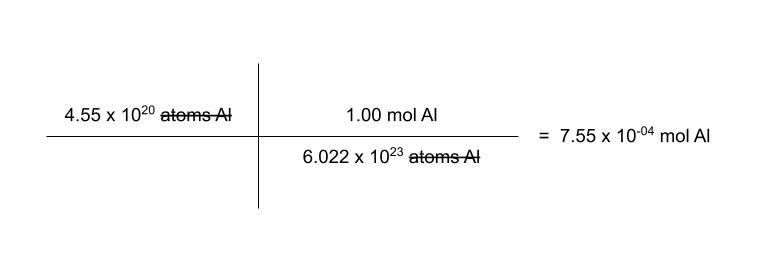
how many moles of aluminum are in 4.55 × 1020 atoms of Al?
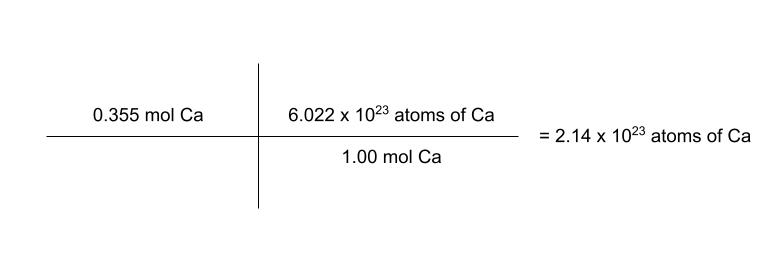
how many atoms are in 0.355 moles of calcium?
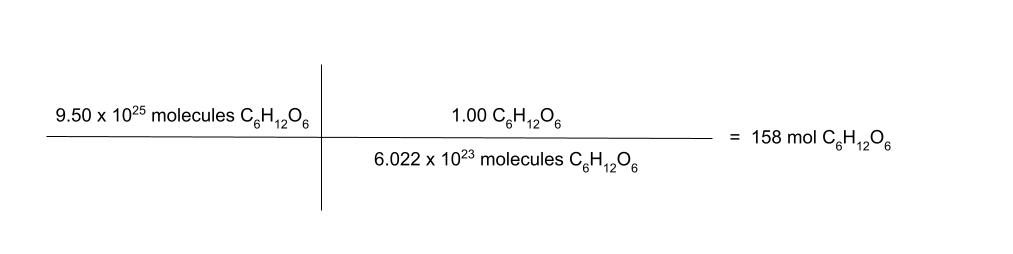
how many moles are in 9.50 × 1025 molecules of glucose? (C6H12O6)
Mass and the Mole
both particles and moles are ways to measure atoms, molecules, ions, or formula units,.
we don’t have a balance that measures in moles and we certainly can’t count out individual atoms. the answer is to find a relationship between number of atoms and mass.
a mole was defined as exactly 12 grams of carbon-12. so for carbon-12, we have this relationship. it turns out that for any element, the mass of the element is the mass of a mole of that element. we call this the molar mass.
helium:
one mole of helium is 4.00 grams of He.
tin:
one mole is 118.71 grams of Sn
oxygen, which occurs as O2
the molar mass is 2(16.00g) which is 32.00g O2
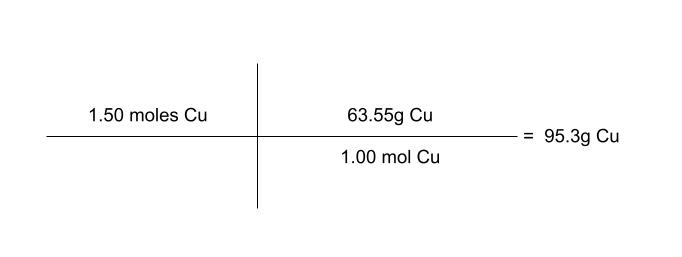
determine the mass of 1.50 moles of copper metal.
a molar mass of a substance is also equal to an Avogadro’s number of particles.
using this, you can convert from mass to number of particles and the reverse
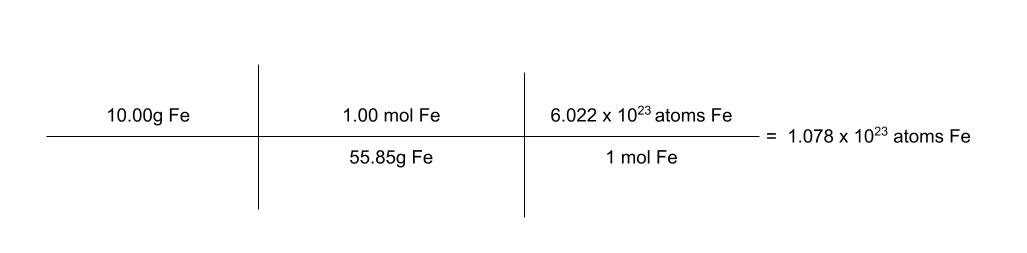
how many atoms of iron are in 10.00 grams of iron?
Moles of Compounds
a chemical formula can be read in two ways.
methane (CH4):
one molecule of methane there is one carbon and four hydrogen atoms
in a mole of methane, there is one mole of carbon and four moles of hydrogen
we can determine the molar mass of methane:
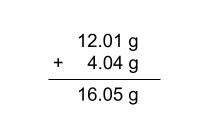 so the molar mass of CH4 is 16.05 g/mol
so the molar mass of CH4 is 16.05 g/mol
CH4
C - one mole of carbon has a mass of 12.01g
H4 - on mole of hydrogen has a mass of 1.01g, so four moles has a mass of 4.04g
determine the mass of 2.55 moles of methane
image
how many molecules are in 1.00 × 103g of methane?
image
find the mass of H2O
image
what is the molar mass of Ca(OH)2?
image
how many formula units of Ca(OH)2 are found in 100.0g of calcium hydroxide? (MM = 74.10 g/mol)
image
how many H atoms are in 125 grams of methane, CH4?
image
Empirical and Molecular Formulas
molecular formula - tells us exactly the number of each element in the formula (C4H8O2)
empirical formula - when chemists cannot find exactly how many atoms of each element were present, but can determine the ratio (C2H4O)
the simplest whole number ratio of the elements in a compound
ex. glucose: molecular formula is C6H12O6 and the empirical formula is CH2O
it is possible for the e and m to be the same, as in sucrose (table sugar), C12H22O11
structural formula - help have an idea of the structure of a molecule
butanoic acid: CH3CH2CH2COOH or CH3(CH2)2COOH
identifying unknown substances:
determine the percent composition of the substance
determine the percentage by mass from a chemical formula. all you need to know is the mass of each element in the compound and the molar mass.
equation:
image
Defining Stoichiometry
the word we use when we are talking about any calculations involving a chemical reaction
stoichiometry → using a balanced equation to determine the ratio of how things react
one important things you must remember about stoichiometry: it depends completely on a balanced chemical equation with the formula of each reactant and product written correctly
Mole to Mole Calculations
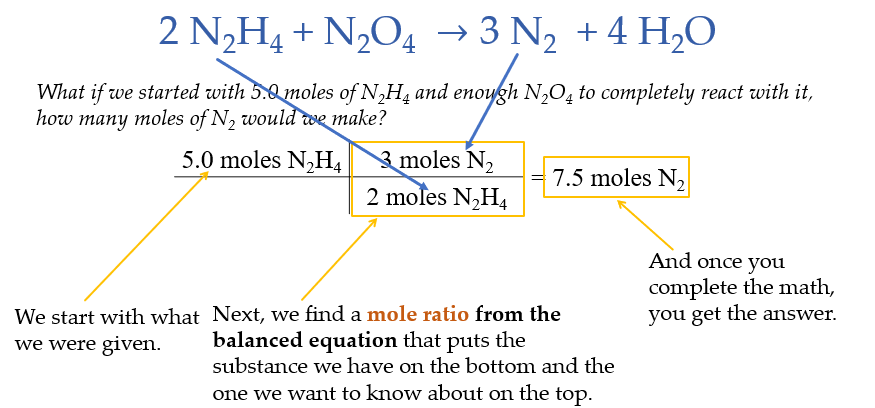
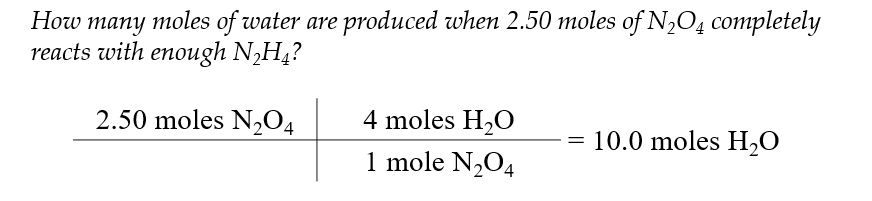
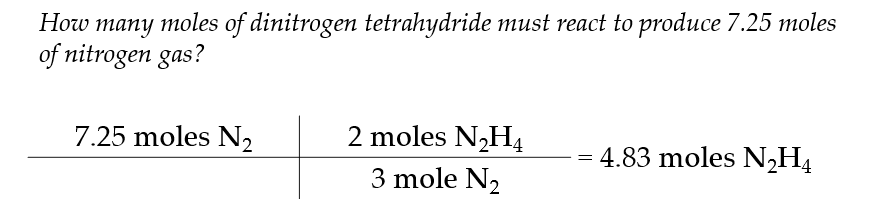 this type of stoichiometry is called a mole to mole conversion
this type of stoichiometry is called a mole to mole conversion
Stoichiometry Calculations With Mass
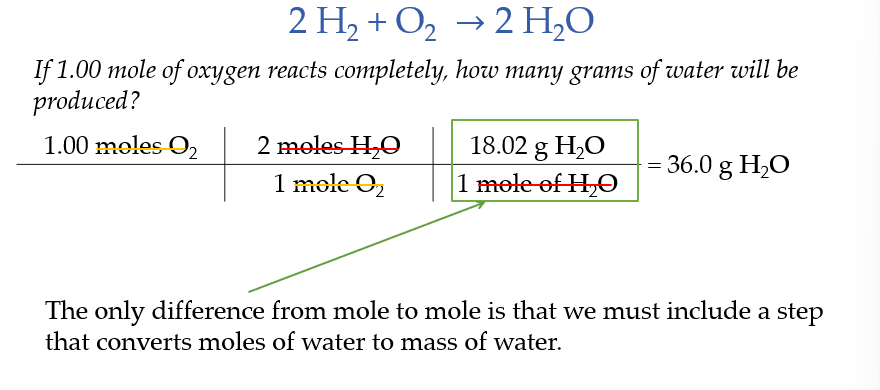
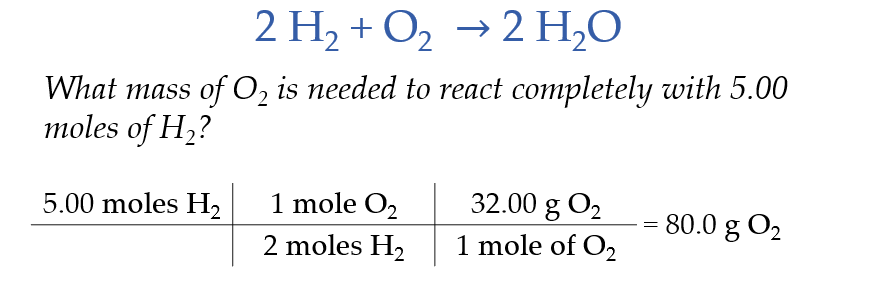
Limiting Reactants
idea of a limiting reactant:
light a campfire, wood is limiting and the oxygen is not; eventually the wood will burn up but the oxygen is limitless
although you had plenty of bread, you ran out of cheese (10 slices of bread; 3 slices of cheese); the cheese was limiting, bread was in excess because we had it left over in the end
in a reaction vessel, we react 20.0 grams of hydrogen with 20.0 grams of nitrogen to make ammonia
A. write a balanced equation for the reaction.
B. determine the limiting reactant.
C. what mass of ammonia will be made?
D. what mass of excess reactant is left after the reaction?
3 H2 + N2 → 2 NH3
![]() we know nitrogen is the limiting reactant which mean hydrogen is in excess. but how much excess hydrogen do we have?
we know nitrogen is the limiting reactant which mean hydrogen is in excess. but how much excess hydrogen do we have?
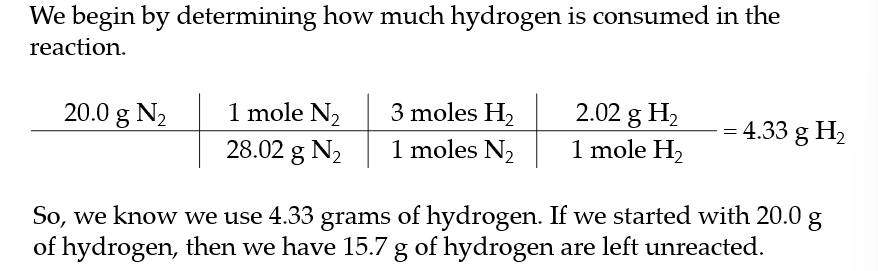
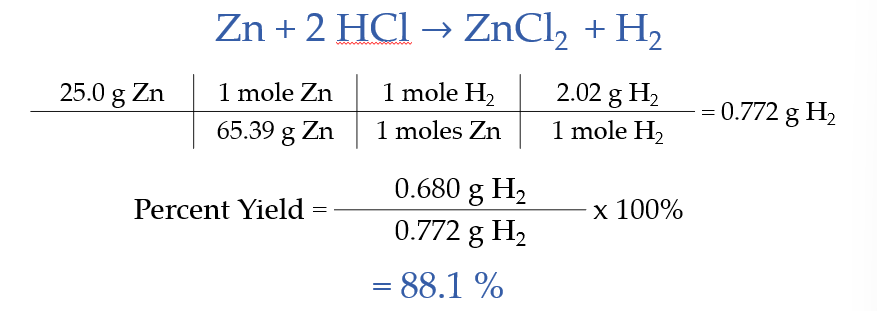
Percent Yield
percent yield - how much of what we thought we should get did we really get in a reaction

zinc reacts with hydrochloric acid producing zinc chloride and hydrogen gas.
A. if 25.0 g of zinc reacts with excess hydrochloric acid, what mass of hydrogen gas will be produced?
B. if the reaction actually yields 0.0680 of hydrogen gas, whet is the percent yield?
Moles, Gases, and the Ideal Gas Law
Boyle’s Law: PV = k ; P1V1 = P2V2 (pressure x volume at one condition equals the pressure x volume in the second condition)
Gay-Lussac’s Law: K = °C + 273.15

Charles’ Law:
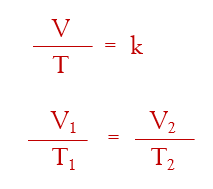
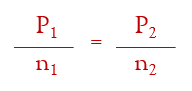
Avogadro’s Law:
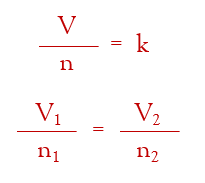
if the temperature and pressure are constant, then one mole of a gas, any gas, would have the same volume. if the temperature and pressure are held constant at Standard Temperature and Pressure (STP = 0.00°C and 1.00 atm), then it turns out that one mole of any gas has the volume of 22.4 Liters.
22.4 L is known as the Molar Volume of a Gas at STP
the Ideal Gas Law:
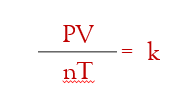 Universal Gas Constant: instead of “k” we represent it with R. so rearranging the equation to remove the fraction, we get
Universal Gas Constant: instead of “k” we represent it with R. so rearranging the equation to remove the fraction, we get
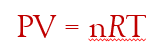 depending on the pressure units used, R equals 0.0821 L⋅atm/mol⋅K or 8.314 L⋅kPa/mol⋅K
depending on the pressure units used, R equals 0.0821 L⋅atm/mol⋅K or 8.314 L⋅kPa/mol⋅K
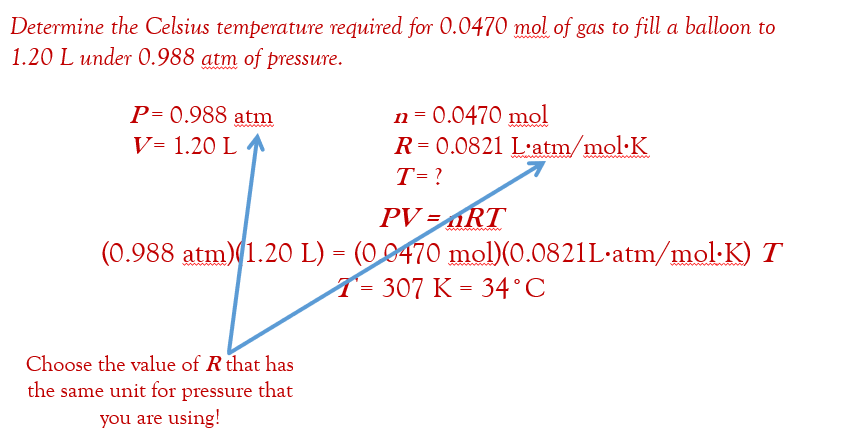
a couple of useful rearrangements of the Ideal Gas Law:
if you need to find the molar mass, M, of the gas…
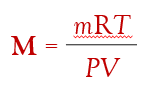 where m is the mass of the gas sample.
where m is the mass of the gas sample.
if you need the density, D, of the gas (remember density is mass per volume)…
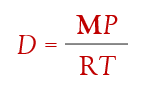
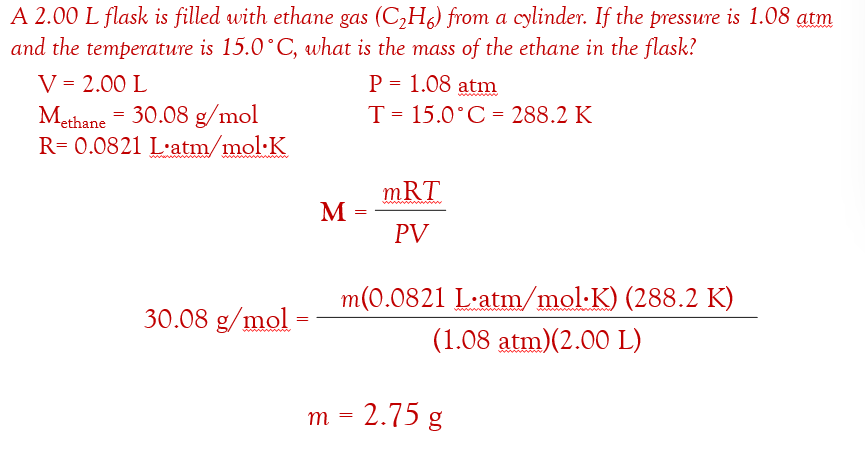
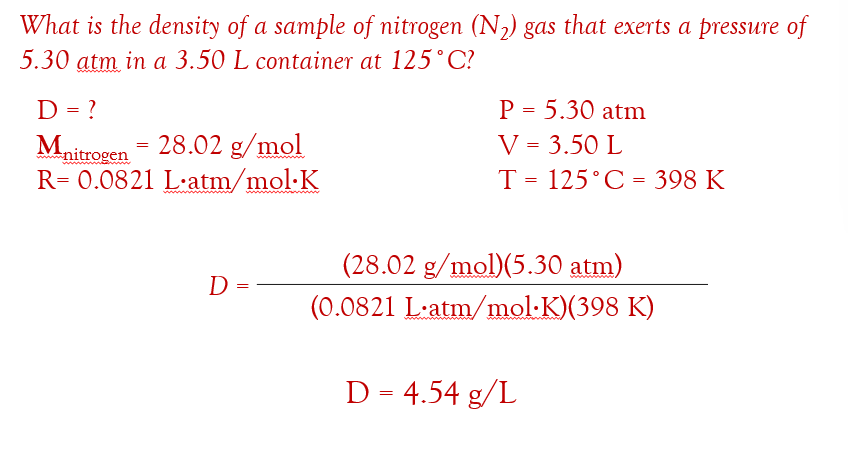
Gas Stoichiometry
volume-volume problems:
ex. ethanol burn to form carbon dioxide and water, according to the equation below. if the pressure and temperature are constant, what volume of CO2 gas will be formed if the reaction uses 6.0L of oxygen?
equation →
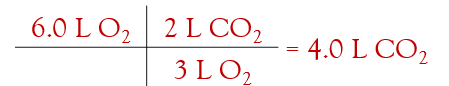 this ratio comes from the balanced equation. the units are whatever you are already using to measure volume so they cancel.
this ratio comes from the balanced equation. the units are whatever you are already using to measure volume so they cancel.
only works if the gas is at STP:
ex. when heated, solid potassium chlorate decomposes to form solid potassium chloride and oxygen gas. if 20.8g of KCLO3 decomposed, how many liters of oxygen gas will form at STP?
equation →
what we know:
mass of KCLO3 = 20.8g
molar mass of KCLO3 = 122.55 g/mol
P = 1.00 atm (STP)
T = 273.15 K (STP)

the gas in any condition:
ex. when heated, solid potassium chlorate decomposes to form solid potassium chloride and oxygen gas. if 20.8g of KCLO3 decomposed, how many liters of oxygen gas will form at 1.50 atm and 373K?