Chemistry S1 Study Guide
CHAPTER 1
What is Chemistry?
Chemistry is the study of matter, including what it is made of, how it behaves, and how it changes. It looks at the properties of substances and the reactions they undergo when mixed together.
What are the steps and applications for the scientific method?
Observation: Identify a problem that could be investigated.
Hypothesis: Make an explanation or prediction for the question based on prior knowledge and research.
Experiment: Design and conduct an experiment to test the hypothesis, ensuring that variables are controlled and data is collected systematically.
Theory vs. Law
Theory: An explanation of the behavior.
Law: A summary of observed (measurable) behavior.
A law tells WHAT happens. A theory is our attempt to EXPLAIN why it happens.
CHAPTER 2
Quantitative vs. Qualitative
Quantitative Observations: These observations are also called a MEASUREMENT and does involve a number with a unit, such as feet or inches. It’s the QUANTITY of something.
Qualitative Observations: These observations DO NOT involve a number. For example, “the sky is blue” or “water is a liquid”.
What is the Scientific Notation?
ALWAYS move the decimal to immediately after the first non-zero digit
Count how many spaces the decimal moved.
If you move the decimal to the RIGHT→ the exponent will become a negative.
If you move the decimal to the LEFT→ the exponent will become a positive.
For example: 0.00012345 will be written as 1.2345 × 10^ -4 (decimal moved 4 places and to the right, indicating it will become a negative exponent.)
For example: 1234500000 will be written as 1.2345 × 10^ 9 (decimal moved 9 places to the left, indicating it will become a positive exponent).
Sig Figs and Rounding Rules
In addition and subtraction, the result should be rounded to the least number of decimal places of any number in the operation.
In multiplication and division, the result should be rounded to the same number of significant figures as the measurement with the least significant figures.
1. Addition and Subtraction
Example 1:
Calculation: 2.356 + 4.1
In this case, 2.356 has three decimal places and 4.1 has one decimal place. Since one decimal place is the least, you round the result:
Result: 2.356 + 4.1 = 6.456 → 6.5
Example 2:
Calculation: 5.6789 - 2.3
Here, 5.6789 has four decimal places and 2.3 has one decimal place. Round to the least:
Result: 5.6789 - 2.3 = 3.3789 → 3.4
2. Multiplication and Division
Example 1:
Calculation: 6.38 x 2.71
Here, 6.38 has three significant figures and 2.71 also has three significant figures. The result should have three significant figures:
Result: 6.38 x 2.71 = 17.2938 → 17.3
Example 2:
Calculation: 5.0 / 1.23
In this case, 5.0 has two significant figures, while 1.23 has three. The result should have two significant figures:
Result: 5.0 / 1.23 = 4.0646 → 4.1
Units (Including S1 Units)
Meter (m) : Length
Kelvin (k) : Thermodynamic temperature
Kilogram (kg) : Mass
Mole (mol) : Amount of a substance
Second (s) : Time
Ampere (A) : Electric Current
Candela (cd) : Luminous Intensity
ETC
Conversions (including length, mass, volume, metric, and temperature)

Length: The metric system allows for straightforward conversions between units. For instance:
1 kilometer (km) = 1000 meters (m)
1 meter = 100 centimeters (cm)
1 centimeter = 10 millimeters (mm)
Mass: Common metric conversions include:
1 kilogram (kg) = 1000 grams (g)
1 gram = 1000 milligrams (mg)
1 metric ton = 1000 kilograms
Volume: Volume can also be converted within the metric system:
1 liter (L) = 1000 milliliters (mL)
1 liter = 1000 cubic centimeters (cm³)
Temperature: Temperature conversions are essential in scientific work. To convert between commonly used temperature scales:
To convert Celsius (°C) to Kelvin (K), use the formula K = °C + 273.15
To convert Kelvin back to Celsius, use the formula °C = K - 273.15 - For instance, 0°C translates to 273.15K.
To convert Celsius to Fahrenheit (°F), use the formula °F = (°C × 9/5) + 32. For example, 0°C equals 32°F.
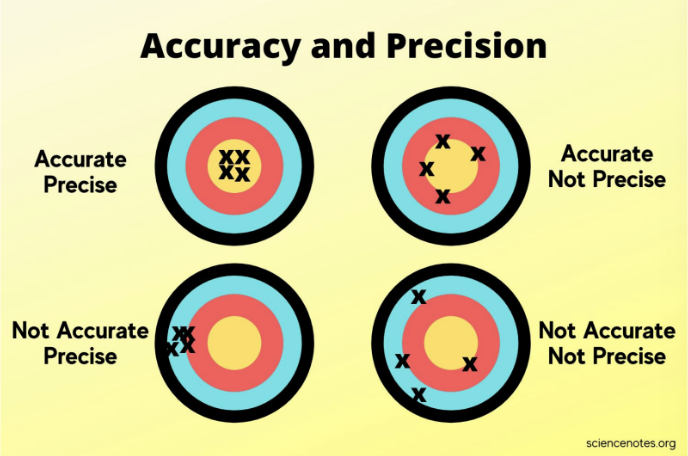
Uncertainty in measurement (1 uncertain digit)
Uncertainty in measurement means that every measurement has a degree of uncertainty, usually shown by one uncertain digit. This represents how confident we are in the measurement, as it may not be perfectly accurate. Understanding uncertainty helps us know how precise a measurement is and how reliable the experimental results are.
If a value is ACCURATE it is true to the actual value.
If a value is PRECISE it is true to itself meaning it is repeatable.
Sig figs can determine the precision or confidence in a measurement.
Dimensional Analysis
● A process through which we can convert one unit of measurement to another
● Used in chemistry problem solving
Ex: How many seconds are in 20 days?
20 days x 24 hrs/1 day
cancel out the days. you will then be left with 20 and 24 hours.
20 days x 24 hrs/1 day x 60 mins/1 hour
the hours will cancel out. you will then be left with 20, 24, and 60 minutes
20 days x 24 hrs/1 day x 60 mins/1 hours x 60 secs/1 min
the mins will cancel out. you will then be left with 20, 24, 60, and 60 seconds
multiply the top and divide by the bottom
the answer will be 1728000 seconds or 1.728 × 10^6.
this will be rounded to 2 × 10^6 seconds.
Density Calculations
Density is defined as the mass of an object divided by its volume. It can be calculated using the formula:
Density (D) = Mass (m) / Volume (V)
To ensure accuracy in density calculations:
Mass should be measured in kilograms (kg) or grams (g).
Volume should be measured in milliliters (mL) or cubic centimeters (cm³).
Example Calculation:
If a substance has a mass of 50 grams and occupies a volume of 10 mL, the density would be:
D = m / V = 50 g / 10 mL = 5 g/mL
Important Notes:
The density of a substance can change with temperature and pressure.
Density can also be used to identify substances; different materials have specific densities.
CHAPTER 3
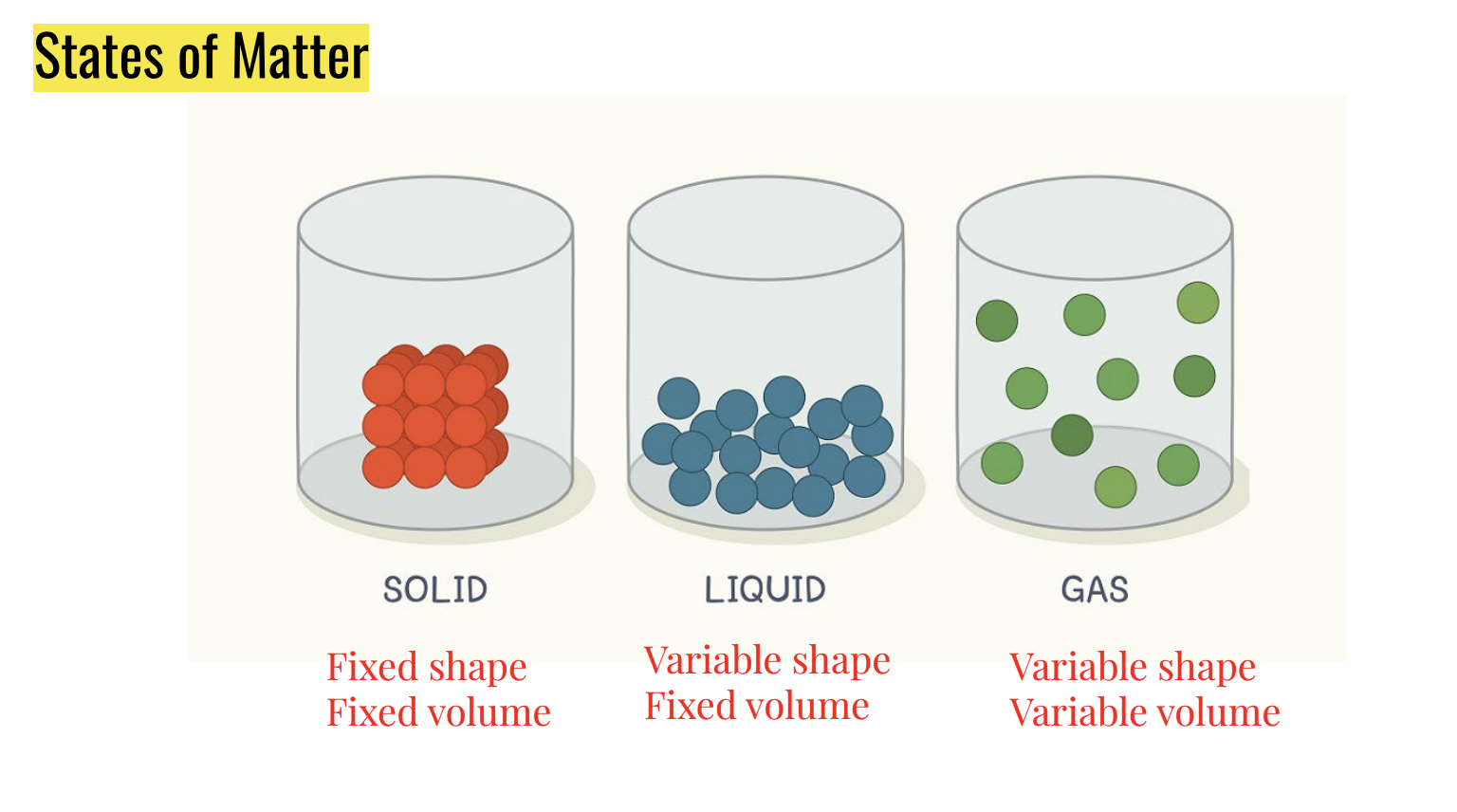
States of Matter
● Everything of which the universe is composed
● Matter must have mass (weight) and occupy space
● Can be solid, liquid, or gas
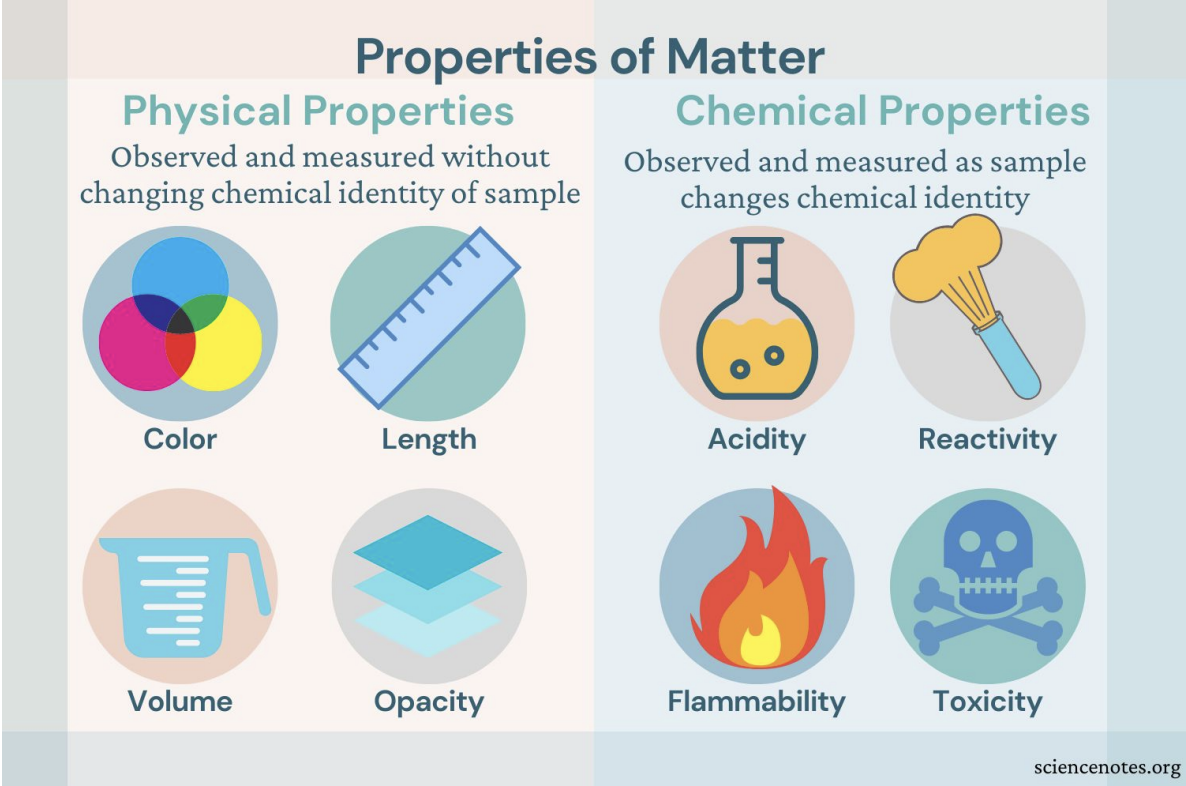
Physical properties describe a substance
Example: Odor, color, volume, state of matter, density, melting point, boiling point
Chemical properties describe how a substance interacts with other substances
○ Typically a substance's ability to form new substances
What are elements?
Elements are substances that cannot be broken down into anything else by chemical means
● Examples are iron, aluminum, oxygen, and hydrogen
What are compounds?
Compounds always have the same composition no matter where we find them.
For example, you may know that water is H2O.
Therefore, it will always have two hydrogens and one oxygen.
What are pure substances?
A pure substance is something that will always have the same composition.
Elements and compounds are considered pure substances.
What is a mixture?
A mixture is something with a variable composition. For example, soil because it can differ in how much of each component is present.
○ A mixture can be separated into two or more pure substances
What is a hetero/homogenous mixture?
A homogeneous mixture is the same throughout and therefore is evenly spread.
Some homogeneous mixtures are also called solutions.
A heterogenous mixture is different throughout and is unevenly spread
What are the 3 types of separation of mixtures?
Evaporation: Liquid is boiled and evaporated out, leaving the solid behind
Filtration: Running a solution/mixture through a porous barrier (such as a coffee filter) to collect the solid as the liquid passes through.
Distillation: Takes advantage of differing boiling points of 2 liquid substances
For example, if you have water (BP 100 deg C) mixed with methanol (BP 65 deg C). Heat to 70 degrees and the methanol will boil out and can be collected.
Can only be used with substances with significantly different boiling points.
CHAPTER 4
Dalton’s Atomic Theory

The Elements
Elements are substances that are unable to be broken down by chemical means
Of the 118 elements, 88 occur naturally
Only 9 elements make up the majority of the Earth which are:
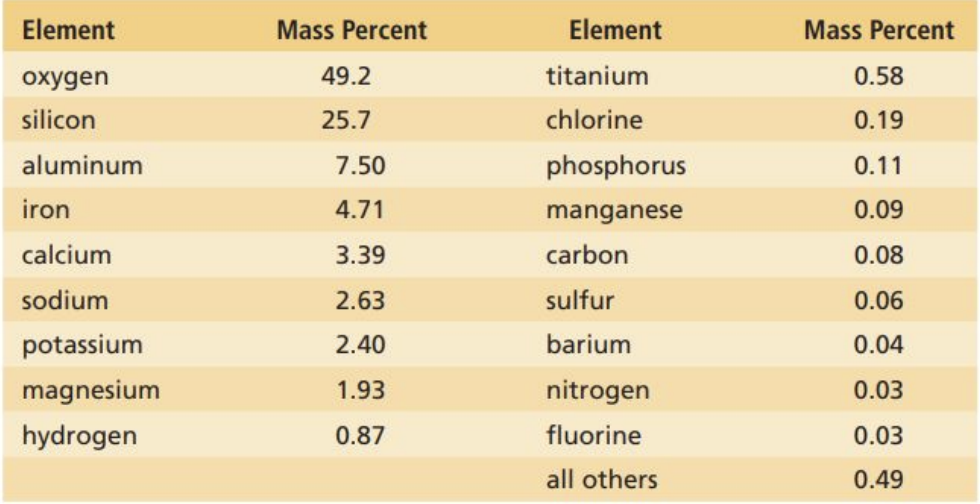
What About the Human Body?
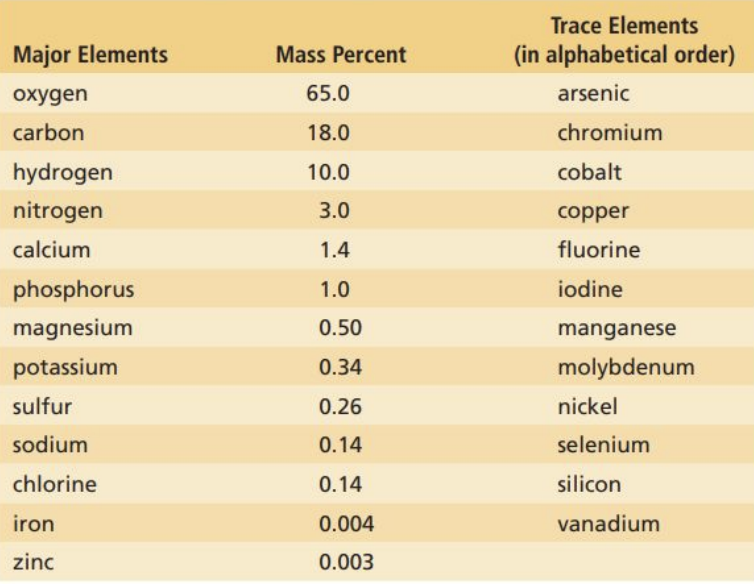
Mass Number vs Atomic Number vs Atomic Mass
1. Mass Number
The total of protons and neutrons in an atom.
Always a whole number.
Example: Oxygen-16 has 8 protons + 8 neutrons = 16.
2. Atomic Mass
The average weight of all versions (isotopes) of an atom.
Usually not a whole number.
Example: Carbon’s atomic mass is 12.01 because of its isotopes.
3. Atomic Number
The number of protons in an atom.
Defines what element it is.
Example: Carbon’s atomic number is 6 (it has 6 protons).
Quick Trick:
Mass Number = Protons + Neutrons
Atomic Number = Protons only
Atomic Mass = Average weight of all atom types.
Different Experiments with Scientists
John Dalton: Dalton thought that atoms are tiny hard spheres that cannot be divided.
J.J Thompson: His conclusion was that all types of atoms must contain these negative particles, now called electrons.
He also knew that atoms as a whole are not positively or negatively charged.
Therefore, the atom must also contain positive particles that balance out the negative charge exactly.
William Thompson: He thought atoms consisted of a uniform positive sphere of matter in which the electrons were embedded like raisins in a pudding (Plum Pudding Model)
Ernest Rutherford: Gold Foil Experiment, concluded that the nucleus contains positive protons.
Him and James Chadwick were able to show that the nuclei of an atom contains a neutral particle as well, which is a neutron.
Writing chemical formulas (from charges and crossover or covalent from a description)
1. Ionic Compounds (Crossover Method)
Write the symbols and charges for the elements.
Example: Sodium (Na⁺) and Chloride (Cl⁻).
Swap the charges and make them subscripts (ignore the signs).
Example: NaCl (charges cancel out).
For Calcium (Ca²⁺) and Chloride (Cl⁻): CaCl₂.
2. Covalent Compounds (Use Prefixes)
Prefixes tell you how many atoms of each element.
Example: "Carbon dioxide" → 1 Carbon (C) and 2 Oxygen (O₂).
"Dinitrogen tetraoxide" → N₂O₄.
Common Prefixes:
Mono- = 1
Di- = 2
Tri- = 3
Tetra- = 4
Penta- = 5
Hexa- = 6
Hepta- = 7
Octo- = 8
Nona- = 9
Deca- = 10
Examples:
Ionic
Mg²⁺ + O²⁻ → MgO.
Al³⁺ + S²⁻ → Al₂S₃.
Covalent
"Sulfur hexafluoride" → SF₆.
"Nitrogen dioxide" → NO₂.
Periodic Table (Groups, Periods, Name of Groups)
Groups, or columns, have similar properties , while periods, or rows, do not.
Group 1: Alkali Metals
Group 2: Alkaline Earth Metals
Group 3-12: Transition Metals
Group 17: Halogens
Group 18: Noble Gases