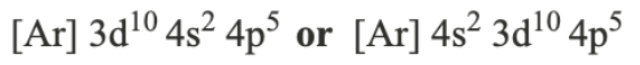Atomic Structure (IB)
The Nuclear Atom
Isotopes
Isotopes: atoms of the same element that have the same number of protons in the nucleus but a different number of neutrons
-differnt mass number
Ex: iodine-131, cobalt-60, lutetium-177
Stable element and isotopes have same chemical properties because same number of electrons
Calculating Relative Atomic Mass
Mass Spectrometer: used to determine the relative atomic masses of elements and is used to determine the structure of organic compounds
Steps to Calculate Relative Atomic Mass:
The sample being studied would be vaporized to form a gas
It is bombarded with high-energy electrons, producing positive ions (+1)
The positive ions are accelerated in an electric field
The positive ions are deflected in a magnetic field depending on the mass ot charge ratio (m/z)
The ions with a higher m/z are deflected less in the magnetic field
The positive ions reach the detector, where they produce a mess spectrum
Electrons in Atom
Atomic Orbitals
Energy Levels and Sublevels
Bohr Model → electrons exist in energy levels
Principle Energy Levels: assigned numbers with n = 1 being the closest to the nucleus and of lowest energy, with the higher numbers being further from the nucleus
The main energy levels ar split into sub-levels: s,p,d,f
Atomic Orbital: a region of space where there is a high possibility of finding an electron
S orbital → spherical
P orbital → dumbbell shaped
D orbital → 4-petal flower
Pauli Exclusion Principle: two electrons cannot have the same quantum number
Number of electrons per main energy level = 2n^2
Heisenberg’s Uncertainty Principle: It is not possible to know, at th same time, the exact position and momentum of an electron. Instead, only a probability can be stated than an electron will be somewher in a given region of space
Electron Configuration
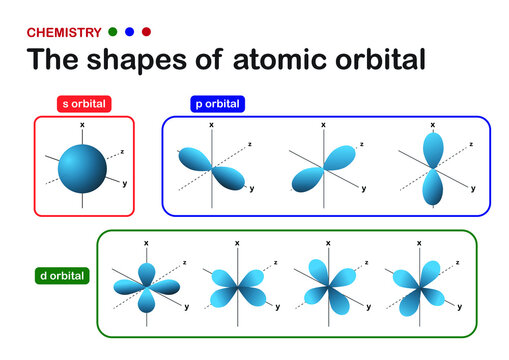
Aufbau Principle: electrons fill atomic orbital of lowest energy first
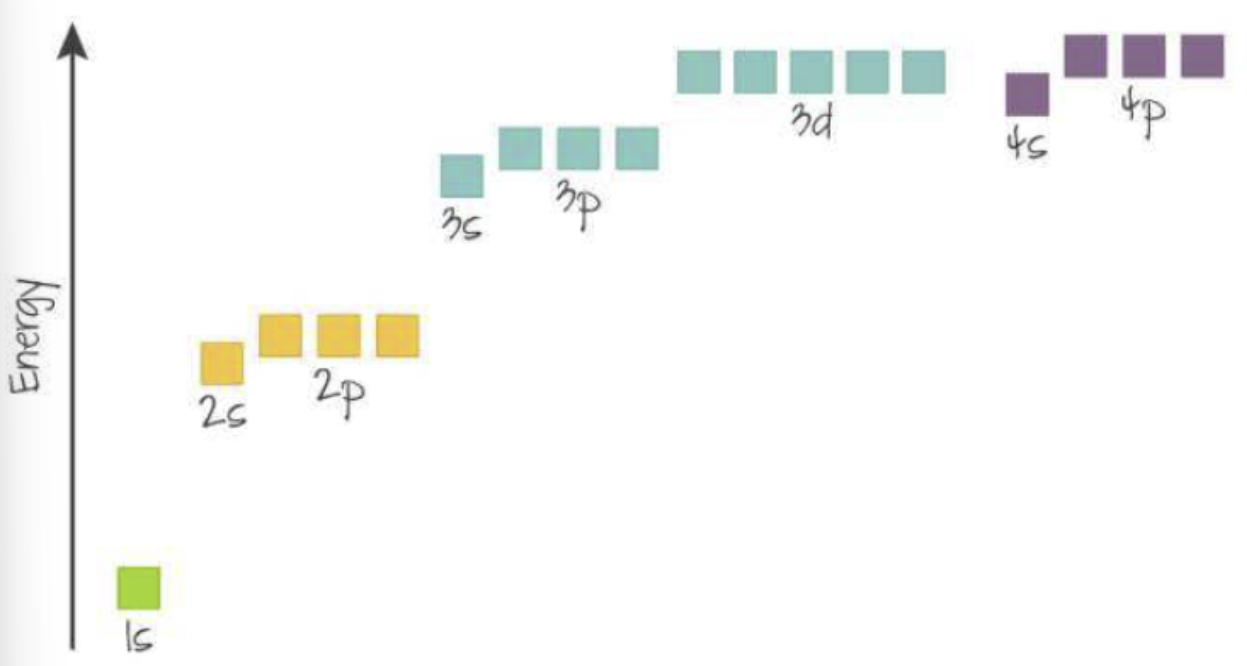
Within a main energy level, s orbitals are of lower energy than p orbitals and therefore fill first
Degenerate orbitals: atomic orbials within a sub-level are of equal enrgy
3 p orbitals (2p, 3p, & 4p sub levels)
5 d orbitals (3d sublevel)
There is an overlap between the 3d and 4s sublevel → 4s is of lower energy and therefore fills first
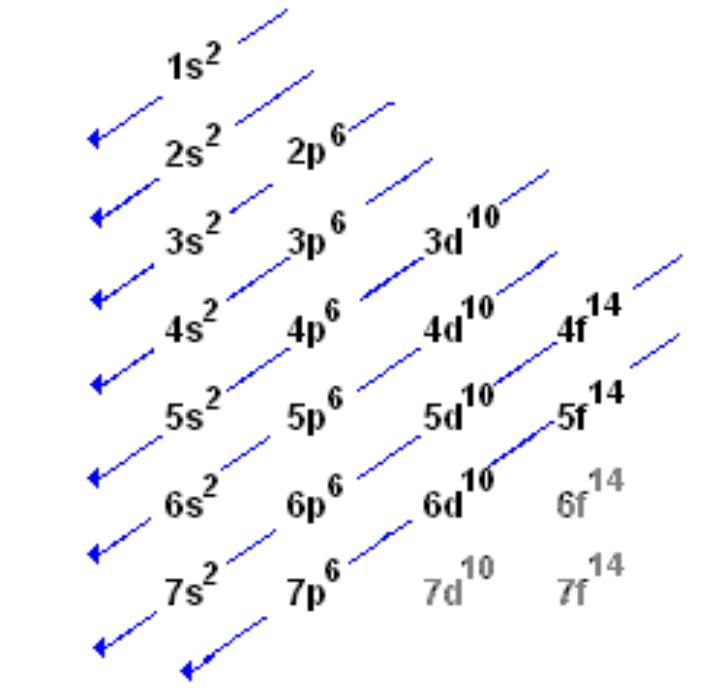
Condensed electron Configuration: a shorthand version of writing the electron configuration for atoms or ions using nobel gasses
Ex: bromine
Exceptions of Aufbau Principle
Chromium
Copper
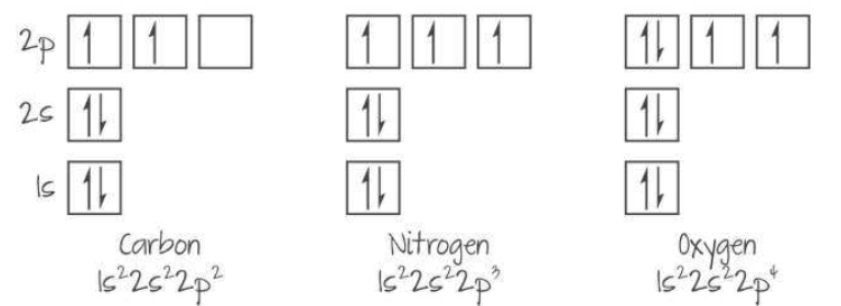
Orbital Diagrams: used to represent electrons in atomic orbitals
Boxes represent atomic orbitals and arrows represent electron
Arrows point opposite direction to represent opposite spins
Only two electrons/arrows per box
Line Spectra
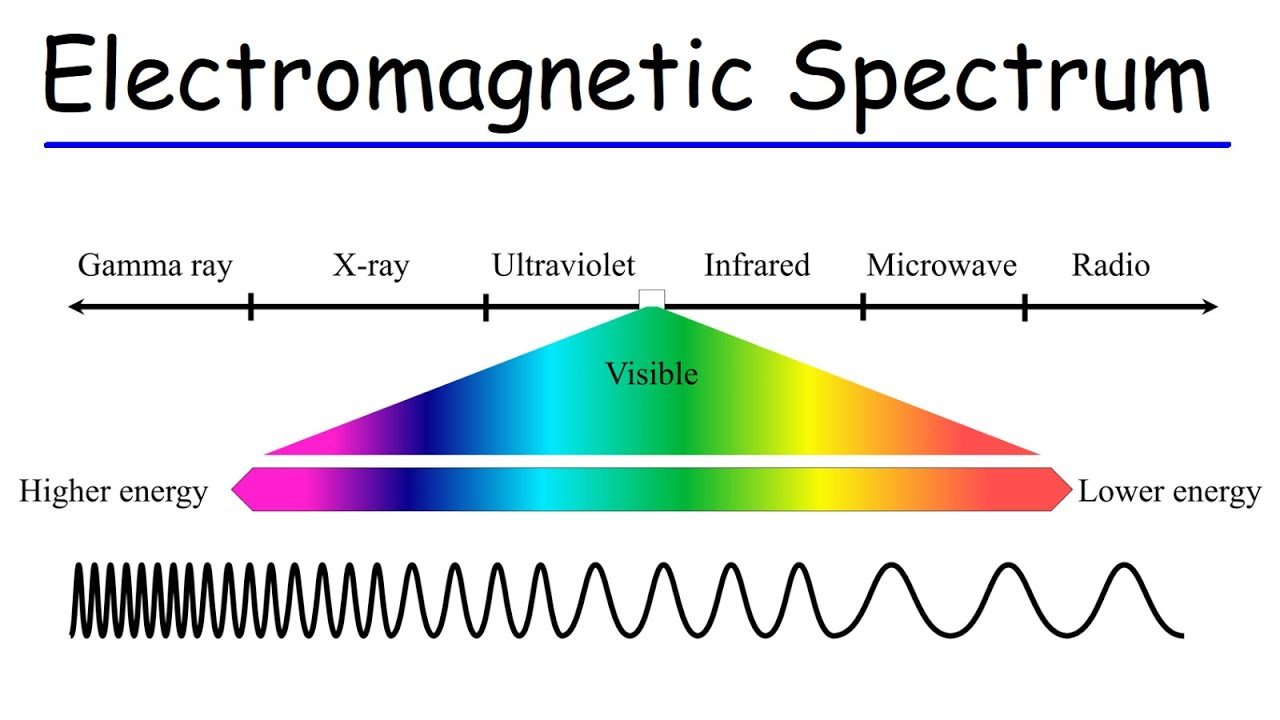
Higher energy → higher frequency → shorter wavelength
Lower energy → lower frequency → longer wavelength
Continuous, Emission & Absorption Line Spectra
The spectrum shows all wavelengths of visible light
Absorption Line Spectra: produced when electrons absorb energy and transition form lower to higher energy levels
Some visible light wavelengths are missing, and are usually shown as black lines on a colored background
Emmision line spectra: produced when electrons emit enryg and tramisition form higher to lower energy levels
The energy emitted when the electrons make these transitions corresponds to the wavelength or frequency of visible light
When moving to the high-energy end of the spectrum (right ot left), lines get closer together
Violet light, having a shorter wavelength than red light, is of higher energy
As electrons absorb/emit energy, they transition between the energy levels
Hydrogen Emission Spectrum
As voltage passes through a sample of hydrogen, electrons are excited to higher energy levels
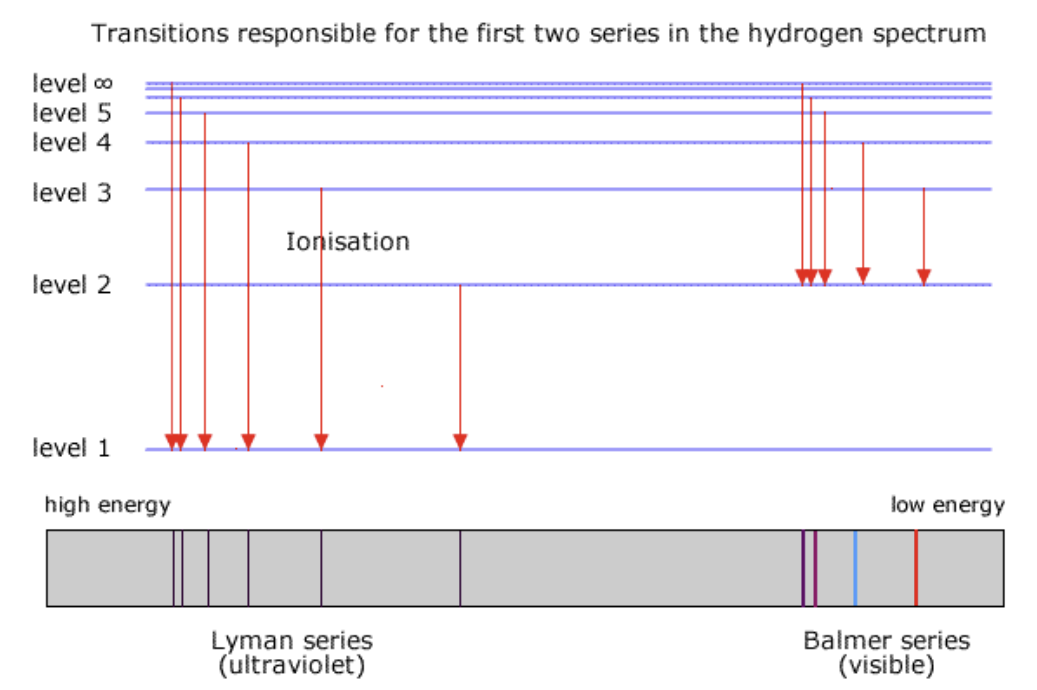
Electron Transitions →
N = 1, Ultraviolet radiation
N = 2, visiblelight
N = 3, infrared radiation
The longerthe arrow, the greater the amount of energy emitted
The electron trnisiton form n=5 to n = 1 emits the largest amount of energy