Periodicity 2 notes
chapter 22.1
Physical properties of period 3 elements
Silicon is a metalloid that can conduct electricity to a certain extent
P,S and Cl are non metals that cannot conduct electricity
Ar is a inert/chemically unreactive noble gas
oxidation state of elements
Na:1+
Mg:2+
Al:3+
Si:4+
P: 3-
S:2-
Cl: 1-
Ar: has no oxidation state
Reactions of sodium and water
2Na(s) + 2H2O(1) 2NaOH(aq) + H2(g)
speed: vigorous
PH:14
Reaction of magnesium and water
Mg(s) + 2H₂O(l)→ Mg(OH)2(aq) + H2(g)
Reaction of magnesium and steam
Mg(s) + H2O(g) MgO(s) + H2(g)
sodium and oxygen symbol equation
2Na(s) + 1/2O(g) → Na₂O(s)
Magnesium and oxygen symbol equation
2Mg(s) + 02(g) → 2MgO(s)
Aluminium and oxygen symbol equation
4Al(s) + 302(g) → 2Al203(s)
formation of silicon dioxide symbol equation
Si(s) + O2(g) → SiO2(s)
phosphorus and oxygen symbol equation
4P(s) + 502(g) P4O10(s)
If there is a lack of oxygen, phosphorus trioxide is formed
Physical properties of the metals in period 3
shiny, conduct electricity, and react with acids to give hydrogen. They are malleable (able to be beaten into sheets) and ductile (able to be drawn into wires). They are good conductors of heat.
Properties of the period 3 non-metals:
They tend to be brittle - they are poor conductors of heat.
in peroxides the oxidation state of oxygen is 1- but in other compounds it’s always 2-
chapter 22.2
type of bonding in aluminium oxide: aluminium is ionic with covalent character because it is a small ion that can distort the electron cloud.
metal oxides (Na2O, MgO and Al2O3) have giant ionic lattices and high melting and boiling points
to predict whether it has ionic character or not, consider the difference in electronegativities
non-metal oxides
silicon dioxide
structure: macromolecular/ giant covalent
MP: High with strong covalent bonds that require lots of energy to break
phosphorous oxide(P4O10)
structure: macromolecular
IMF: van der Waals and permanent dipole-dipole forces
MP: Low
states at room temperature, 298 K, sulfur dioxide and sulfur trioxide
PO10 > SO3 > SO₂
Why do the melting points get weaker across the period?
There is an increase in intermolecular van der Waals forces between larger molecules
structures of oxo acids:
phosphoric acid:
P: has 5 electrons
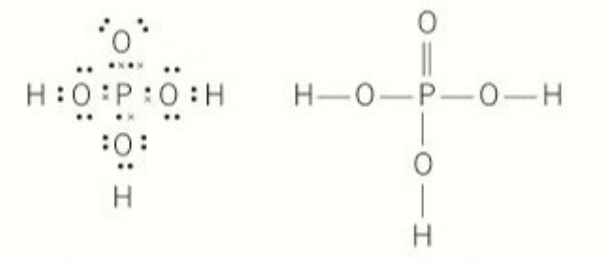
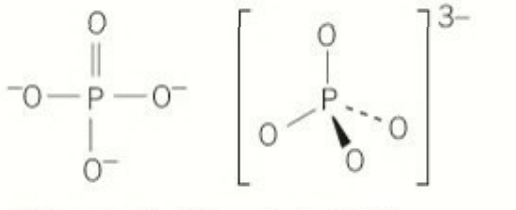
shape of all 3 oxo ions: a perfect tetrahedron that has delocalisation
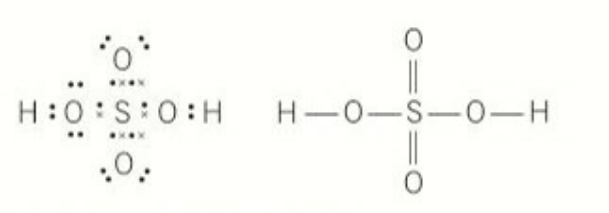
sulfuric acid
number of electrons: 6
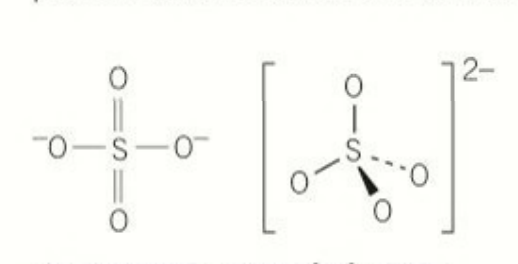
bond angle of an ideal tetrahedron: 109.5
lengths of bonds in the phosphorus oxo anion: they are all the same length
number of electrons in phosphorus’ outer main shell: 5
Number of electrons in sulfur’s outer main shell: 10 electrons
number of electrons sulfur has in its outer main shell in the sulfate (IV) anion: 10
Na₂O(s) + H2O(l) →2Na+ (aq) + 2OH-(aq) pH of solution~14
MgO(s) + H₂O(1) →Mg(OH)2(s)Mg2+(aq) + 2OH-(aq) 10-13
Which metal oxides are insoluble: aluminium oxide and silicon oxide
acidic oxides:
PO(s) + 6H2O(l) →4H3PO4 (aq)
H3PO4(aq)→ H+ (aq) + H2PO(aq)(reversible reaction)
SO2(g) + H₂O(l) →H2SO3(aq)partially dissociates in water
H₂SO3(aq)H+(aq) + HSO3 (aq)
SO3(g) + H2O(l) H2SO(aq) H+(aq) + HSO(aq) violient reaction
Key points of the behaviour of oxides:
Na and Mg are ions
Na2O is a strong base that dissolves to form an alkaline solution
MgO is less soluble and is a weaker base
Aluminium oxide cannot be separated
General trend: Solutions of the oxides of the elements go from alkaline to acidic across the period.
reactions with acids:
Na2O (s) + H2SO4 (aq) → Na2 SO (aq) + H2O(l)
MgO(s) + 2HCl(aq) → MgCl2(aq) + H₂O(l)
Al₂O3(s) + 6HCl(aq) → 2AICl3 (aq) + 3H₂O(l)
Al₂O3(s) + 2NaOH(aq) + 3H2O(l)→ 2NaAl(OH))aq)
Properties of silicon dioxide: weak acid
SiO₂(s) + 2NaOH(aq)→ Na₂SiO3(aq) + H₂O(l)
Si02[s) + Ca0(I) →CaSi03 (l)
phosphorous pentoxide
H3PO4(aq) + NaOH(aq)→ NaH2PO4(aq) + H₂0(l)
NaH PO (aq) + NaOH(aq)→ Na2HPO4(aq) + H₂O(l)
Na2HPO4 (aq) + NaOH(aq)→ Na3PO4(aq) + H₂O(l)
Overall equation:
3NaOH(aq) + H3PO4(aq)Na3PO4(aq) + 3H2O(l)
SO2(aq) + NaOH(aq) → NaHSO3 (aq)
Ca0(s) + SO2(g]→ CaS03(s)
metal chlorides
NaCl and MgCl2
Aluminium chloride structure:
The aluminium has only six electrons in its outer main shell, and it forms a dimer in which coordinate covalent bonds form between lone pairs of electrons on the chlorine atoms and the electron-deficient aluminium atoms.
Silicon tetrachloride:
holds 18 electrons in the outer main shell, SiCl4
structure: gaint ionic lattice
aluminium chloride in water
AlCl3 (s) +3H2O (1) → Al(OH)3(s) + 3H+(aq) + 3Cl-(aq)
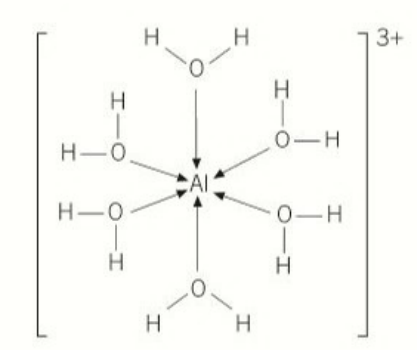
[Al(H₂O)] 3+(aq) →[Al(H₂O) (OH)] 2+(aq) + H+(aq)
SiCl(l) + 2H2O(l)→ SiO2(s) + 4H+(aq) + 4Cl-(aq)
PCI5(s) + 4H2O(1)→H,PO(aq) + 5H+(aq) + 5Cl-(aq)