Unit 6: Thermochemistry
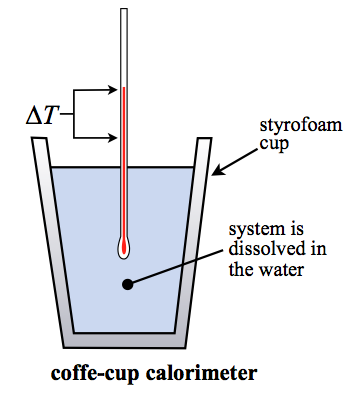
1) Coffee Cup Calorimeter - Insulated system, reaction in a solution
Constant volume and pressure (ΔV = ΔP = 0)
Cannot expand or contract
Liquids are incompressible
***If it involved a liquid or a solid, it maintains a constant volume and pressure.
Work = 0
ΔH = ΔE = Q
2) Piston Calorimeter - Gas Forming Reactions
Piston changes volume (variable volume but constant P)
Ex: Gasoline engine
ΔV≠0, ΔP = 0
ΔH = ΔE + PΔV = Q - PΔV + PΔV = Q —> ΔH = Q

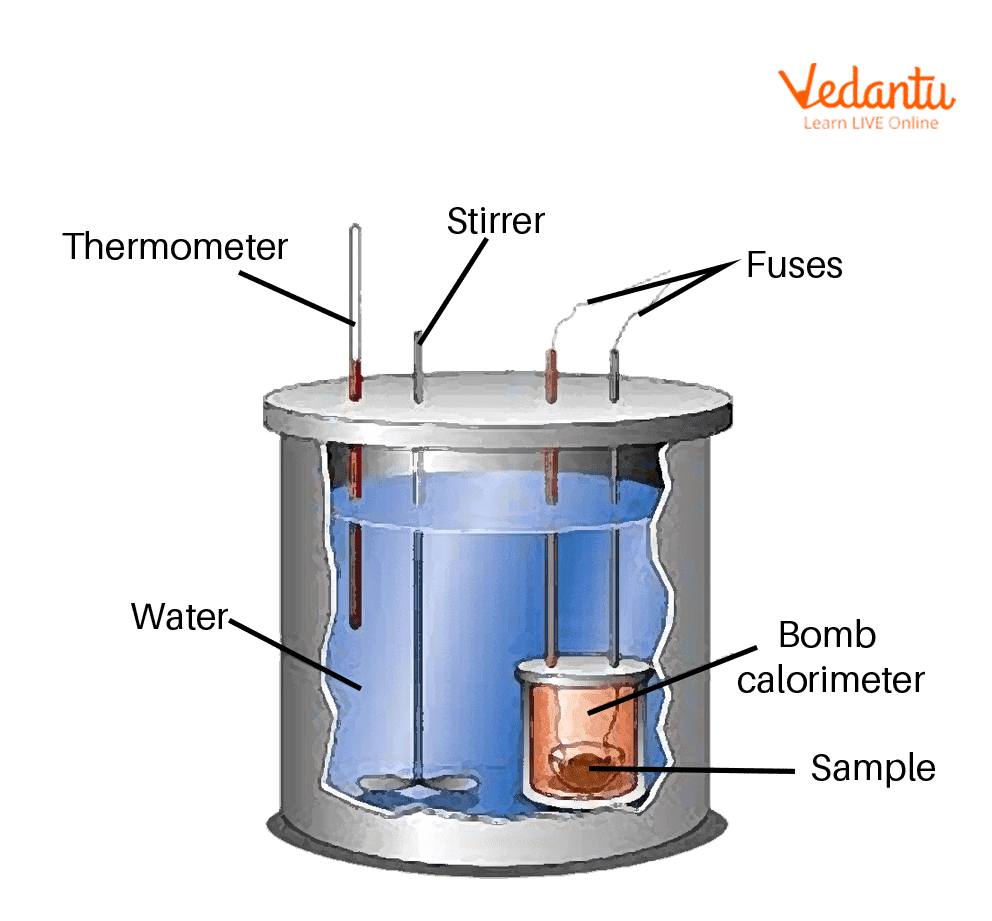
3) Bomb Calorimeter - Gas forming reaction
Constant V but varying P
ΔV = 0, ΔP ≠ 0
Measuring temp (C) of H2O
ΔE = Q
ΔH = Q + VΔP
*High Specific Heat = lower thermal conductivity
Example: A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol.
Qrxn = Qcal + QH2O
Qcal = 837 J/ºC * (33.20 - 25.00) = 6862.4 J = 6.86 kJ
QH2O = 1200 × 4.184 x (33.20 - 25.00) = 41170.56 J = 41.17 kJ
- Q rxn = 6.862 + 41.17 = 48.032 kJ (the reaction is negative because it releases heat, as evident by the fact that the water increased in temperature
1.000 g C8H18/114.23 g/mol = 0.00875 moles of C8H18
-48.032/0.00875 moles = -5489.4 kJ/mol
Example: 0.5060 grams of liquid cyclohexanol undergo complete combustion in a bomb calorimeter. The calorimeter assembly has a heat capacity of 827 J/ºC and contains exactly 1000 grams of water. What is the final temperature it the initial water temperature is 24.98 C? The heat of combustion is -3727 kJ/mol
-(-3727 kJ/mol) = Qcal + QH2O = 827(Tf - 24.98) + 1000 g * 4.184 (Tf - 24.98)
0.5060 grams of C6H12OH/100.12 = 0.005054 moles
3727 × 0.005054 × 1000 = 827(Tf - 24.98) + 1000 g * 4.184 (Tf - 24.98)
Tf = 28.74 C
Example: A coffee-cup calorimeter having a heat capacity of 472 J/ºC is used to measure the heat evolved when the following aqueous solutions, both inititally at 22.6 C are mixed: 100 grams of a solution containing 6.62 g of lead (II) nitrate, and 100.0 grams of solution containing 6.00 grams of sodium iodide. The final temperature is 24.2 C. Assume that the specific heat of the mixture is the same as that for water (4.184 J/gºC). Find the H reaction
1) Balanced equation: Pb(NO3)2 (aq) + 2NaI (aq) —> PbI2(s) + 2NaNO3
Moles of Pb(NO3)2 = 6.62/331.2 = 0.02
Moles of NaI = 6.00/149.89 = 0.04
No Limiting Reactant
-Qrxn = Qcal + QH2O = 472 (24.2 - 22.6) + 200.0 g * 4.184 * (24.2 - 22.6) = 2094.08 J = 2.094 kJ
Qrxn = -2.094 (negative because it releases energy, as evident by the increase in temperature of the solution/surrounding)
Hrxn = Qrxn/moles = -2.094 kJ/0.02 moles = -104.7 kJ/mol
Heat of Formation
Formation reaction: synthesis of a compound from its elements in standard conditions (25 C, 1 atm, 1M)
Example: Formation reaction of Ethanol (C2H5OH)
2 C(s) + 3H2(g) + ½ O2(g) —> C2H5OH (l) ΔHf = -277.7 kJ/mol
C2H5OH (l) ΔHf = -277.7 kJ/mol
C2H5OH (g) ΔHf = -235.7 kJ/mol
Different heat of formation for gas and liquid. It takes energy to turn vaporize the liquid into a gas (Heat of vaporization)
Hf (l) + Hvap = Hf(g)
-277.7 + x = -235.7 —> x = 42.6 kJ/mol
Hrxn = ∑nproducts * Hf - ∑nreactants * Hf
Products - Reactants

Solid Ice: C = 2.09
Water: 4.184
Gas: 2.02
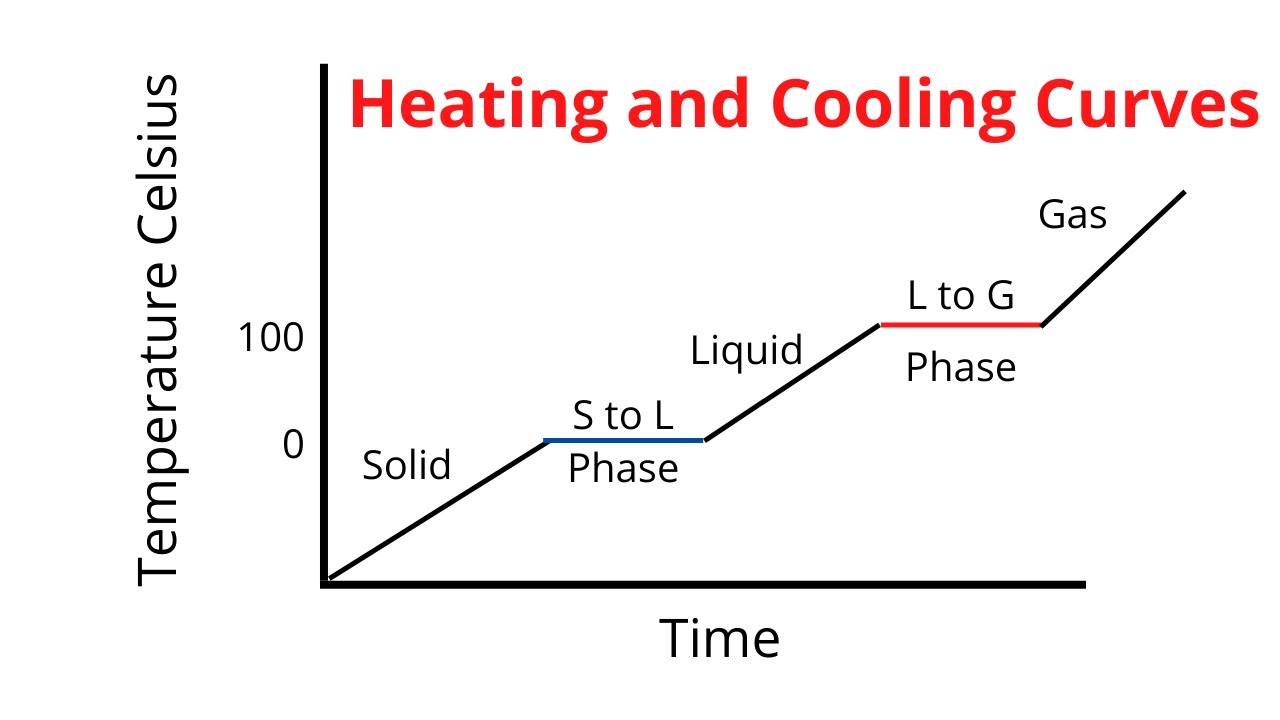
Heat of vaporization: 2260 J/g
Heat of Fusion: 334 J/g
Hvap>HFus because it takes more energy to completely break the bonds between water molecule to bring them from a liquid to a gas. When going from solid to liquid, the bonds are only partially broken/loosened.
*Important: For the heat of fusion, it is positive when it is an endothermic process. When a substance freezes, it releases the energy so the correct formula would be -m x Hfus.
For heat of vaporization: it is positive when it is an endothermic process. When vapor condenses, it releases the energy so it would be -m x Hvap
Example: How much heat is required to cool 25 g of water vapor from 130 degree C to -5 degree C?
Q = mCAT - mHvap + mCAT - mHfus + mCAT
Q = 25(2.02 * (100-130) - 2260 + 4.184 (0-100) - 334 + 2.09 (-5-0))
Q = -77,086.25 J = -77.09 kJ
Energy
ΔE = Q + (-PΔV)
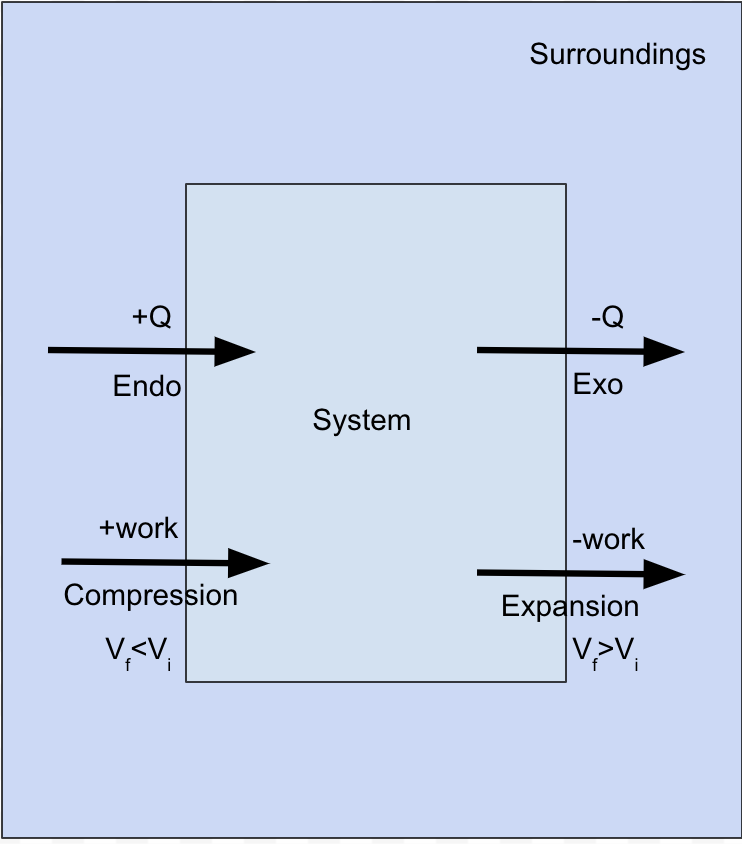
Increase ΔE = putting heat into system and compressing it
Decrease ΔE = releasing heat and expanding
UNITS: 1 L* atm = 101.3 Joules = 101.3 × 10^-3 kJ
Topics covered on the Test:
1) Calorimetry (Cup and Bomb)
-Qrxn = Qcal+QH2O/surrounding
Hrxn = Qrxn/moles of limiting reactant
QH2O<QCal because the calorimeter absorbs some of the heat
2) Mass-Heat stoichiometry
3) Hess’s Law, ΔHf, and Bond dissociation energy (use these to calculate Hrxn)
Hess’s Law: Reversing a reaction —> flip the sign of the Hf
Using ΔHf: products - reactants
Using BDE: Bonds broken-Bonds formed
Breaking bonds is positive enthalpy and forming bonds is negative enthalpy
4) ΔE = Q + (-PΔV)
Look at above diagram
5) Potential Energy Diagrams

Diagram A is endothermic (+ΔH) and diagram B is exothermic (-ΔH)
Reactants to top of the graph is the activation energy
Adding a catalyst decreases the activation (does not affect the reacts or products)
6) Mixing Hot and Cold
Condensation, freezing = exothermic, melting, freezing = endothermic
**Usually positive means heat absorbed and negative means heat released.
+ΔH = endothermic = treated as reactant
-ΔH = Exothermic = treated as product

 Knowt
Knowt