C3 - Chemicals of the Natural Environment
3.1 - How are the atoms held together in a metal?
What are metals?
Metals are elements that are good conductors of electricity and heat. Metals are strong, hard, solid at room temperature (except for mercury), ductile, and malleable.
Ductility - how capable a material is of being pulled into thin sheets/wires without breaking.
Malleability - how capable a material is of being pressed into a new shape without breaking or returning to its original shape.
Metallic Structure
Since metals are generally solid, the atoms in metals are tightly packed.
Specifically, in metals, metal ions are closely packed together in a regular arrangement, held together by metallic bonds.
Metallic Bonding
Metallic bonds are formed by the electrostatic attraction between opposite charges.
Metal is made up of electrons surrounding positive metal ions. The difference between these charges creates a bunch of bonds between all the ions.
Metallic Structure and Bonding Help Explain the Properties of Metal
Metallic bonds are strong, so the structure is held tightly together, resulting in the metal’s hardness.
Because metallic bonds are so strong, a large amount of energy is required to break them. This is why metals have a high melting point.
Layers of metal ions are able to slide over each other in response to an applied force, so metals are malleable and ductile.
Electrons between the metal ions are free to move, which helps metals be good conductors of heat and electricity.
3.2 - How are metals with different reactivities extracted?
The Reactivity Series of Metals
The reactivity series of metals is a list of metals from most reactive to least reactive.
When metals react with non-metals, they form ionic compounds by losing electrons. The easier it is for a metal to lose electrons, the more reactive it is.
You can use the reactivity series to make predictions about how quickly a metal reacts.
Below is the reactivity series of metals (with hydrogen shown for comparison). Remember, metals are listed from most reactive to least.
Metal | Reaction with water | Reaction with dilute acids |
|---|---|---|
K | Violent with cold water | Violent |
Na | Violent with cold water | Violent |
Ca | Slow with cold water, rapid with steam | Rapid |
Mg | Slow with cold water, rapid with steam | Rapid |
Al | Typically no reaction | Rapid |
Zn | Typically no reaction | Slow |
Fe | Rusts slowly | Slow |
(H) | - | - |
Cu | No reaction | No reaction |
Ag | No reaction | No reaction |
Au | No reaction | No reaction |
When metal reacts with water, hydrogen and a resulting metal hydroxide are formed.
Example: Ca + 2H2O → Ca(OH)2 + H2
When metal reacts with a dilute acid, hydrogen and a resulting salt are formed.
Example: Mg + 2HCl → MgCl2 + H2
Metals that are less reactive than hydrogen in the reactivity series will not react with dilute acids. On the other hand, the more reactive the metal, the quicker the reaction will occur.
Displacement Reactions
In a displacement reaction, one or more atoms takes the place of another atom or set of atoms.
Single displacement: A + BC → B + AC
Double displacement: AB + CD → AD + CB
In displacement reactions, a more reactive metal can take the place of a less reactive metal in its compound.
Example: Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Iron is more reactive than copper, so it is able to displace the copper from the copper sulfate. As a result, iron sulfate forms and copper forms a solid precipitate.
To write an ionic equation for displacement reactions,
Break each reactant and product into their respective ions.
If an ion appears on both sides of the equation, it is a spectator ion (not involved in the reaction) and the equation can be rewritten without it.
In the end, the equation will show the ions that are involved in the reaction and the resulting products.
Example: Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Write the dissociated ions: Fe (s) + Cu2+ (aq) + SO42- (aq) → Fe2+ + SO42- (aq) + Cu (s)
Remove spectator ions: Fe (s) + Cu2+ (aq) → Fe2+ + Cu (s)
Extracting Metals
Ore - a rock that a metal can be extracted from. An ore can be low or high grade. Low grade ores contain only a small percentage of the metal, while high grade ores contain a large percentage.
There are different ways to extract metals, depending on the metal’s position in the reactivity series.
Most metals can be extracted using electrolysis, but this method uses large amounts of energy.
Metals that are less reactive than carbon can be extracted from its oxide form by being heated with carbon.
Remember displacement reactions. In this case, carbon will displace the metal from its compound, separating the metal for extraction.
Carbon is in between aluminum and zinc on the reactivity series.
As a general rule: metals that are more reactive than carbon can be extracted through electrolysis, and metals that are less reactive than carbon are extracted by heating with carbon.
Alternative Methods of Metal Extraction (Higher)
These methods can also be referred to as biological methods since they use living organisms. The two methods covered in this section are phytoextraction and bioleaching.
Phytoextraction - Plants absorb the ions that are present in the soil they are planted in. This includes metal ions. Currently, this method is not used for commercial purposes, but it can be used to remove toxic metals from soil. It is theorized that when higher grade ores have run out, plants can be burned to extract metal. The resulting ash would have higher metal concentration than the soil.
Bioleaching - Certain types of bacteria have been found to be able to oxidize metal oxide ores. These can be found in spoil heaps of old mines and have been found to work best in acidic conditions. When the spoil heaps are sprayed with acid, oxidation occurs, and the metal can be extracted.
3.3 - What are electrolytes and what happens during electrolysis?
Electrolytes - ionic compounds that are either dissolved in water or molten
Molten substances are liquid, formed by adding heat to a solid
In either of these states, ions are free to move throughout the liquid.
Electrolysis - the process of breaking down electrolytes using an electric current. The resulting free ions are attracted to the charged electrodes connected to the source of the current.
Electricity is the flow of electrons.
Cations - positively charged ions
Example: Na+, H+, Mg2+
Anions - negatively charged ions
Example: Cl-, OH-, SO42-
Electrode - A conductor connected to the source of the current and submerged in the electrolyte substance to create a circuit. There are two oppositely charged electrodes.
Cathode - the negatively charged electrode that attracts positively charged ions (cations)
Anode - the positively charged electrode that attracts negatively charged ions (anions).
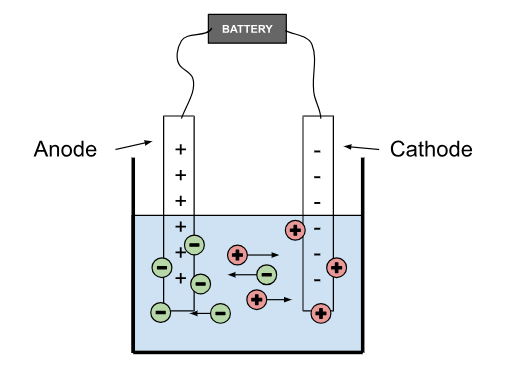
Products of Electrolysis
Cations go to the cathode, and anions go to the anode. When ions get in contact with electrodes, they form different molecules.
When cations (+) reach the cathode (-), they gain electrons
Example: At the cathode, Na+ would become Na
When anions (-) reach the anode (+), they lose electrons
Example: At the anode, Cl- would become Cl
Example: In the electrolysis of molten aluminum oxide, what products are formed at the electrodes?
Molten aluminum oxide has freely flowing aluminum ions (Al3+) and oxide ions (O2-). The aluminum ions move towards the cathode and gain electrons, while the oxide ions move towards the anode and lose electrons.
Answer: Aluminum forms at the cathode and oxygen forms at the anode.
Electrolysis of Acidified Water
Water contains H+ and OH-. If water is acidified (mixed with a small amount of acid), the H+ ions go to the cathode and form hydrogen gas (H2). The OH- ions go to the anode and form oxygen gas (O2).
Remember that at the electrodes, anions lose electrons and cations gain electrons.
Electrolysis of Dissolved Ionic Compounds
A dissolved ionic compound solution contains H+ and OH- ions from the water, as well as anions and cations from the compound. The ions compete to gain/lose electrons at the electrode.
If the metal cation is less reactive than hydrogen, the metal will form at the cathode. If the metal cation is more reactive than hydrogen, then hydrogen will form.
Refer to “Reactivity Series of Metals” below
Oxygen is produced at the anode unless the dissolved compound contains halide ions (Cl-, Br-, I-).
Oxidation and Reduction in Electrolysis
Half equation (or half-reaction) - an equation that shows the change that one substance undergoes in a chemical reaction.
Example: Ca2+ + 2e- → Ca
Half equations also must be balanced. In a balanced half equation, there are the same amount of each atom on either side of the equation. The net charge also has to be the same on either side.
In electrolysis, half-reactions are used to show the reactions that occur at each of the electrodes.
Oxidation-Reduction (or Redox) reaction - A reaction in which electrons are transferred between chemical species.
Oxidation and reduction are typically shown as half-reactions. A redox reaction shows the combined reaction of a pair of oxidation/reduction half-reactions.
Example of a redox reaction: 2Na + Cl2 → 2Na+ + 2Cl-
Oxidation - the process of a substance gaining oxygen, or losing electrons, during a chemical reaction.
Example of an oxidation half-reaction: Na → Na+ + e-
Reduction - the process of a substance losing oxygen, or gaining electrons, during a chemical reaction.
Example of a reduction half-reaction: Cl2 + 2e- → 2Cl-
One way students remember the difference between oxidation and reduction is by using the acronym “OILRIG”.
Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons)
In electrolysis…
Oxidation occurs at the anode
Remember that the anode is the positive electrode, so anions (negative ions) will lose electrons here.
Reduction occurs at the cathode
Remember that the cathode is the negative electrode, so cations (positive ions) will gain electrons here.
3.4 - Why is crude oil important as a source of new materials?
Crude Oil
Crude oil is a natural petroleum product that is extracted from the earth and turned into many different products, like gasoline. On a molecular level, crude oil is complex and made of hydrocarbons (compounds that contain only hydrogen and carbon).
Crude oil is used to produce fuels and feedstock for the petrochemical industry.
Note: fuels produced from crude oil are known as fossil fuels, and are a non-renewable resource. This means that once all of it is used up, it can’t be replaced.
Properties of Hydrocarbons
Hydrocarbons are made of only hydrogen and carbon atoms. These molecules vary in size, with some containing only a few atoms total, and others containing long chains of atoms.
Hydrocarbons have very strong chemical bonds between atoms, but weaker forces of attraction between molecules (intermolecular force).
Longer hydrocarbon molecules have a stronger intermolecular force.
Substances made of longer hydrocarbons require more energy for molecules to be pulled apart. This results in lower volatility, lower flammability, and a higher boiling point.
Volatility - how easily a substance evaporates
Flammability - how easily a substance can ignite
Boiling point - the temperature at which a substance goes from its liquid phase to its gas phase
Shorter hydrocarbons have weaker intermolecular forces, so they are more volatile, highly flammable, and boil at a lower temperature.
Alkanes
Crude oils are made of hydrocarbons that mostly come from one group: the alkanes.
Alkanes:
Have the same general formula
General formula: CnH2n+2
Have similar chemical properties, but vary with physical properties.
Four common alkanes are methane, ethane, propane, and butane. Their structures and formulas are shown below:
Alkane | Molecular formula | Structure |
|---|---|---|
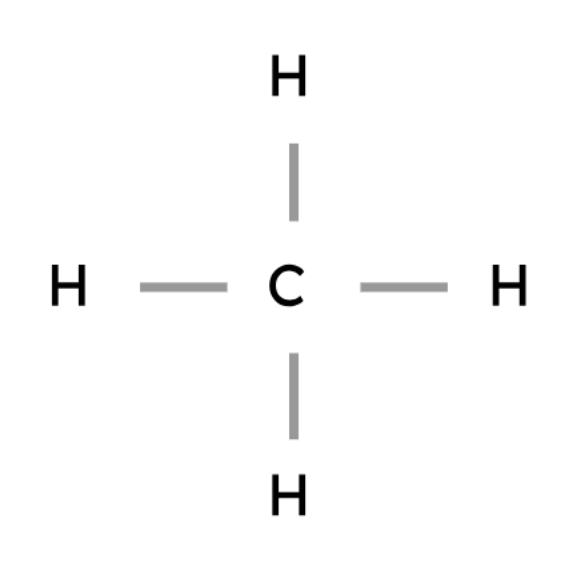
Methane | CH4 |
|
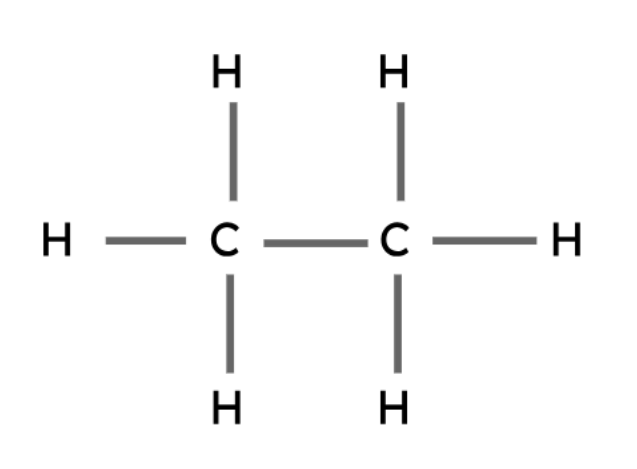
Ethane | C2H6 |
|
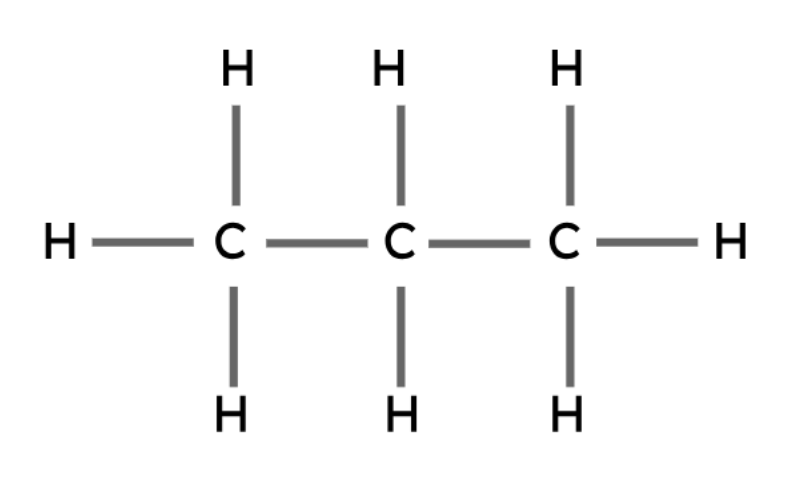
Propane | C3H8 |
|
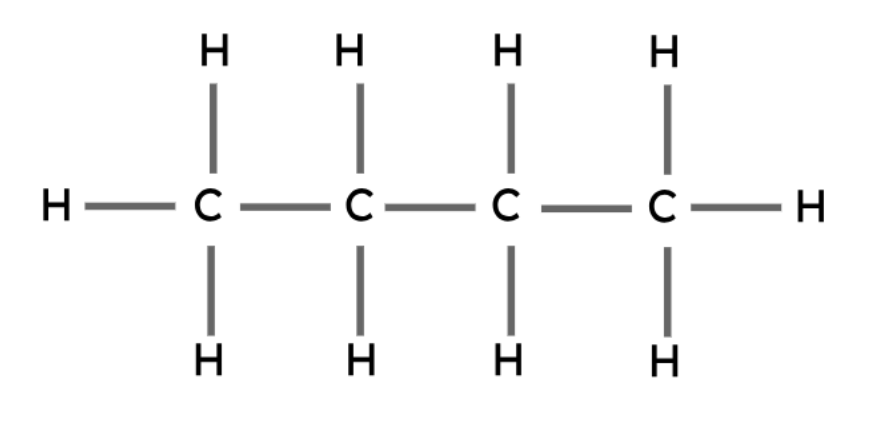
Butane | C4H10 |
|
Cracking Hydrocarbons
Long chain hydrocarbons can be cracked, or broken down into smaller hydrocarbon molecules. The starting hydrocarbon is an alkane, and the broken down products include alkanes and alkenes.
Alkenes are still hydrocarbons, but they contain a carbon-carbon double bond.
To crack hydrocarbons, they are heated so that their vapors pass over a catalyst. This results in their covalent bonds breaking and reforming into smaller hydrocarbons.
Catalyst - a substance that changes the rate of a chemical reaction.
Example: Hexane (C6H14) can be cracked to form ethene (C2H4, an alkene) and butane (C4H10, an alkane).
C6H14 → C2H4 + C4H10
Empirical Formula
Sometimes, empirical formulas are used to show the simplest ratio of each type of atom present in a molecule. This is done by looking at the subscripts in a molecular formula.
Example: Hexane has a molecular formula of C6H14. This means there are 6 carbon atoms and 14 hydrogen atoms.
The ratio of carbon to hydrogen can be written as 6:14, but can be simplified to 3:7.
Rewriting a molecular formula with the simplest ratio gives the empirical formula. The empirical formula for hexane is C3H7
More Organic Chemistry
Alcohols
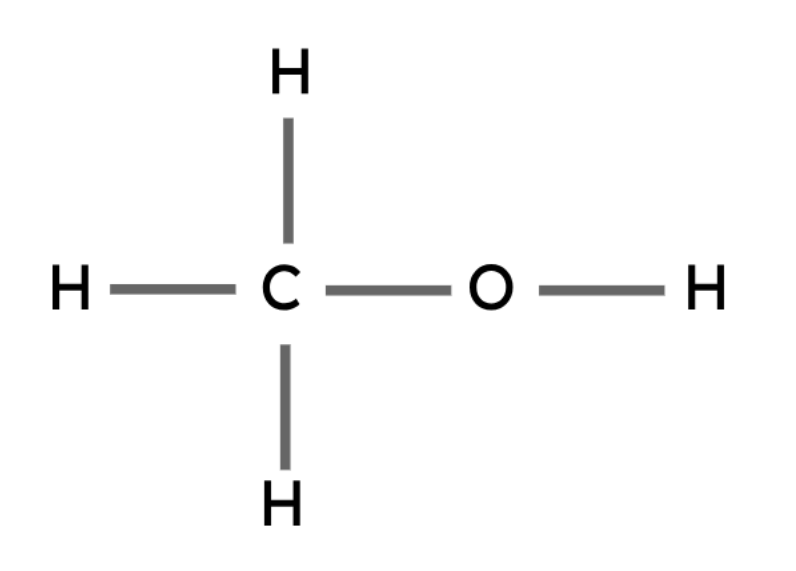
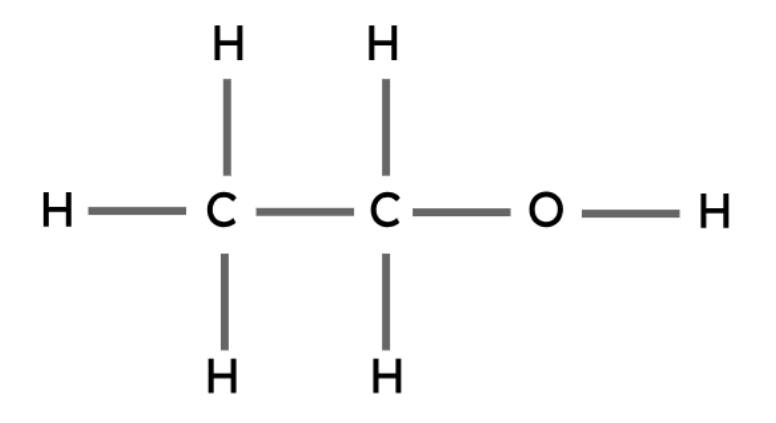
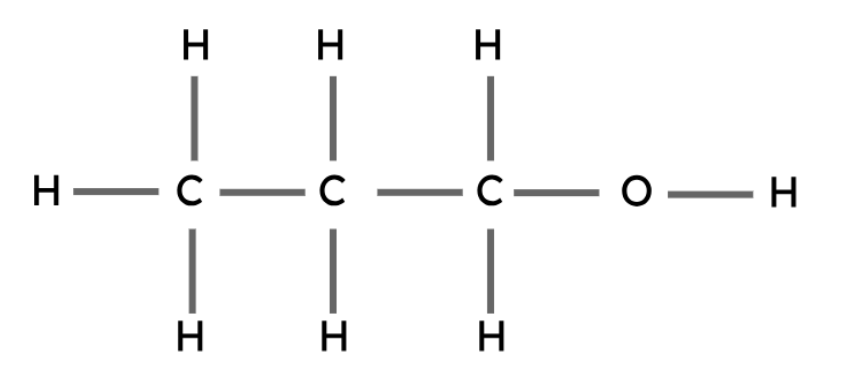
Alcohols are another type of organic compound. These compounds all have the same general formula and contain a hydroxyl group (-OH).
The hydroxyl group is the functional group in alcohols. Functional groups are the groups of atoms that determine the chemical properties of an organic compound.
General formula: CnH2n+1OH
The chart below shows three common alcohols and their structures:
Alcohol | Molecular formula | Structure |
|---|---|---|
Methanol | CH3OH |
|
Ethanol | C2H5OH |
|
Propanol | C3H7OH |
|
Carboxylic Acids
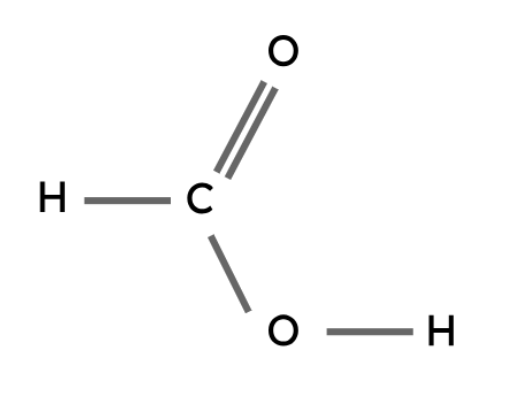
Like the other types of compounds described above, carboxylic acids also follow a general formula and have similar chemical properties.
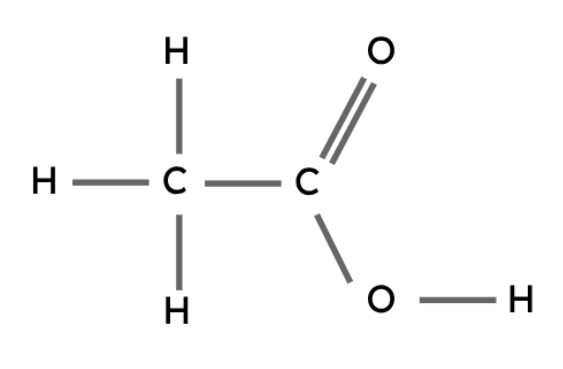
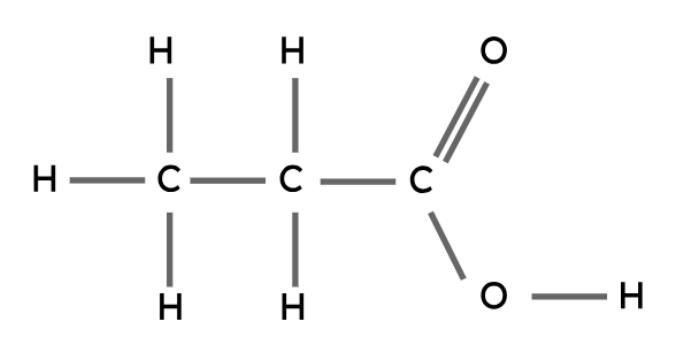
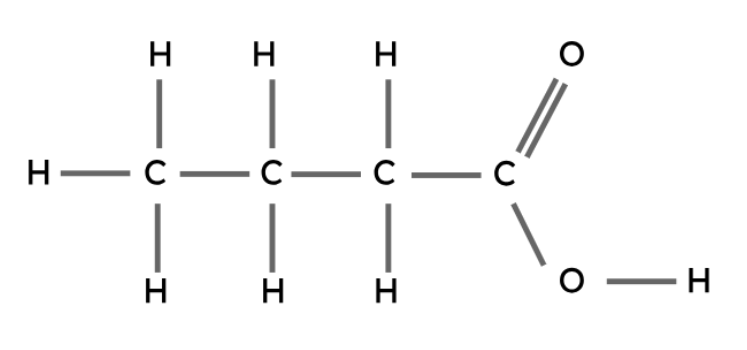
The carboxyl group (-COOH) is the functional group in carboxylic acids.
General formula: CnH2n+1COOH
The table below shows four common carboxylic acids and their structures:
Carboxylic Acid | Molecular formula | Structure |
|---|---|---|
Methanoic acid | HCOOH |
|
Ethanoic acid | CH3COOH |
|
Propanoic acid | CH3CH2COOH |
|
Butanoic acid | CH3CH2CH2COOH |
|
Carboxylic acids share many properties with acids, including the tendency to:
React with metals to produce a salt and hydrogen
React with bases to produce water and a salt
Dissolve in water to form solutions with pH < 7
 Knowt
Knowt