Science Chemistry Molecular Substance and Intermolecular Forces
Lesson 1: Introduction to molecular substances
Substances are either a metal, non- metal or both
There are 2 types of Molecules
- Molecular Elements
All elemental molecules are made up of atoms of a single element. This means they contain 2 atoms of the same element and are therefore called… diatomic e.g. Hydrogen (H2)
Molecular Compounds
Molecules of compounds have atoms from different elements. e.g. Water (H2O)
Binary molecular compounds are those that contain exactly 2 elements
Greek prefixes are used to indicate the number of atoms of each element present
There are 4 rules of naming binary covalent compounds:
- First element is named in full
- Second element is shortened and the -ide suffix is added
- Use a prefix, note mono- is never used for the first element
- If the name of the 2nd element begins with a vowel and the prefix for the name ends with a or o these letters are dropped.
| mono- | 1 |
|---|---|
| di- | 2 |
| tri- | 3 |
| tetra- | 4 |
| penta- | 5 |
| hexa- | 6 |
| hepta- | 7 |
| octa- | 8 |
| nona- | 9 |
| deca- | 10 |
Lesson 2: Lewis structure of atoms and simple molecules
Electron Dot Diagram of Atoms
Electron dot diagrams only show the outer-shell electrons in atoms and molecules. It shows the number of electrons available for bonding
Remember:
- For groups 1 and 2, the number of valence electrons is the group number
- For groups 13 - 18, the valence electrons is the group number minus 10
Unpaired electrons = Bonding electrons
Paired electrons = Lone pairs
Covalent Bonding
Covalent bonding = Is a chemical bond that involves sharing electron pairs between atoms. They’re formed when non-metallic atoms combine together to form covalent substances
Atoms share electrons in covalent bonding to form a full outer shell
When 2 non-metal atoms bond together they form a covalent bond sharing electrons to make the molecule more stable
Electron Dot Diagram of Molecules
Summary
- Atoms achieve a full outer shell to become …? (stable)
- Covalent bonds form bonds between non metals by … electrons (sharing)
- Electrons are shared … … (in pairs)
- Each shared pair represents a … … (covalent bond)
- Covalent bonds can be, single, double or triple bonds
Lesson 3: Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory is used to determine the shape of molecules
The key idea is that the valence electron pairs repel each other. Therefore they are arranged as far apart as possible.
There are 5 basic molecular shapes
| Lewis structure | Bonding regions | Lone pairs | Molecule shape | Bond angle | Shape name |
|---|---|---|---|---|---|
| 2 | 0 | 180∘ | Linear | ||
| 2 | 1 | 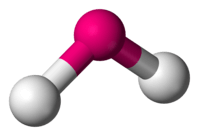 | 116∘ | Bent | |
| 2 | 2 |  | 104.5∘ | Bent | |
 | 3 | 0 | 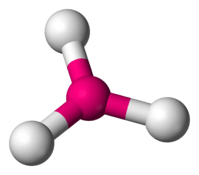 | 120∘ | Trigonal planar |
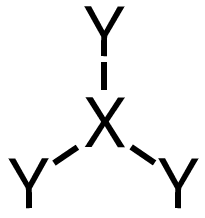
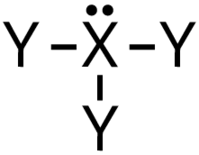 | 3 | 1 | 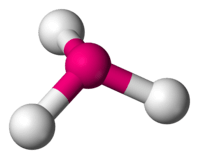 | 107∘ | Trigonal pyramidal |
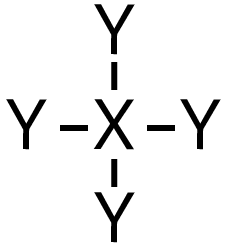 | 4 | 0 | 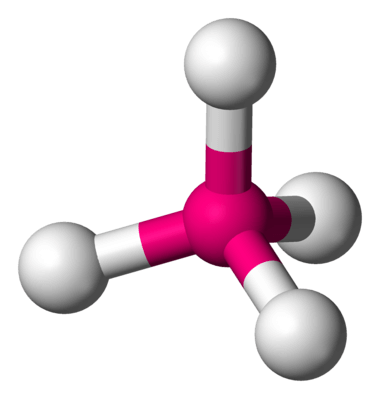 | 109∘ | Tetrahedral |
Lesson 4: Representations of Molecules
Different type of molecule diagrams
- The electron dot diagram helps to determine the type of bonds but does not show the size or shape of molecules.
- The valence structure can show single, double or triple bonds using lines. Lone pairs are shown with dots or lines. Does not show size or shape of molecules.
- In structural formulas bonding pairs are shown with lines/dashes. Lone pairs are left out. 3D structure may be required.
- The ball and stick model displays the shape of the molecule including single, double and triple bonds It shows the shape but not the size of atoms.
- The space-filling model shows the whole atom including the electron cloud. Shows the size and position of atoms but not types of bonds or angles.
Lesson 5: Polarity of Covalent Bonds
Electronegativity = A measure of how strongly the atoms attract electrons in chemical bonds
Non polar occurs
- Difference is less or equal to 0.4 electronegativity
- When atoms are the same element
- Similar electron negativity e.g. Cl and N(3.16-3.04 = 0.12)
- Bonding electrons are equally distributed
- Equal distribution of electrons
Polar occurs
- Difference between 0.4-1.7 electronegativity
- Different electronegativities therefore the atoms won’t be shared equally
- The bigger the electronegativity difference the more polar
Ionic bond is when its greater than 1.7
Lesson 6: Polarity of Covalent Molecules
Polar molecules:
One end has a permanent partial negative charge and the other partial positive. These charged ends are called dipoles (meaning 2 poles)
Polarity of diatomic molecules
Recap: What is a Diatomic molecule? When only 2 atoms are connected by a covalent bond. Which can be single, double or triple bond.
The polarity of a diatomic molecule is the same as the polarity of the covalent bond
Same as above
| Non Polar: Occurs if 2 atoms are the same element. Neither end attracts the electrons more. No dipole is formed | Polar: A difference in electronegativity between the 2 atoms. Atom with highest electronegativity attracts the electrons. Dipole formed |
|---|
Polyatomic molecules
Recap: Are made up of 3 or more atoms.
An unbalanced distribution of electrons can result in a dipole same as diatomic molecule
To determine the polarity, note 2 things:
What are the 2 things? Polarity of bonds and the symmetry of molecules
- The polarity of the bonds
If at least 1 covalent bond is polar the electrons are unevenly distributed
hence consider the entire distribution of electrons to determine if polar
- The symmetry of the molecule
Asymmetric: Distribution of electrons will be unbalanced and a dipole will form
Symmetric: Distribution of electrons will be balanced and no dipole is formed
Lesson 7: Intermolecular Forces - Part A
| Intramolecular Bonds | Intermolecular Bonds |
|---|---|
| Holds 2 atoms together within a molecule e.g. Covalent bonding or ionic bonding (haven’t really learnt that) | Holds 2 molecules together e.g. Hydrogen bonding, dipole-dipole bonding, dispersion bonding(lesson 8) |
| - Bonds inside the molecule break or form: Chemical changes | - The individual molecules don’t change but their interaction with neighbouring molecules do: Physical changes |
Strength of intermolecular bonds/forces:
- The stronger the bonds the more energy need to break them
- Therefore the stronger the bond the higher the melting and boiling points
<<Dipole - dipole force<<
ONLY occurs between TWO POLAR MOLECULES
PERMANENT partial POSITIVE charge of ONE molecule will be attracted to the NEGATIVE of another
THE STRENGTH of these interactions determines PHYSICAL CHANGES
<<Hydrogen Bond<<
Is a SPECIAL type of DIPOLE-DIPOLE bonding that occurs between 2 MOLECULES it is 10X STRONGER
1 MOLECULE must contain a HYDROGEN atom that is directly/covalently bonded to an OXYGEN, NITROGEN OR FLUORINE ATOM
THE (N,O,F) must contain a LONE pair
Water!!!!!!!!!! Mermaid!!!!!!!!!!!!!!!
Can form 4 hydrogen bonds with 4 other water molecules
Properties:
High melting and boiling points due to the H-bonding so more energy is needed to break the bonds
Liquid form = water molecules are closely packed together but are not fixed in place
Solid form = The water molecules form 4 hydrogen bonds with its neighbouring molecules and forms a HEXAGONAL CRYSTALLINE LATTICE. Therefore the molecules are further spread out from each other resulting in the expansion of ice
Water requires more energy to be absorbed for an increase in temperature compared to other fluids. This is = Heat capacity for water
Lesson 8: Intermolecular Forces - Part B
Dispersion force!!!!!!!!!!!!!
Dispersion forces exist between ALL molecular compounds, regardless of polarity
Formation of temporary dipole:
- For non polar molecules the electrons are distributed around the molecule evenly. However as they are constantly moving there are points in time where more electrons are on one side creating a more negative and more positive side.
- When a molecule forms a temporary dipole it can encourage neighbouring molecules to make one too. They align their temporary dipoles with opposite attractions forming the dispersion force. This is similar to dipole-dipole forces but is generally weaker due to it being temporary
Strength of dispersion force:
- Molecules with larger molecular mass = more electrons
- Which means it is easier to produce a larger temporary imbalance of electrons = stronger temporary dipole bonds
- Therefore the strength of dispersion forces increases as the molecular mass increases
- Usually DF is weaker than D-D and H-D. However in really large molecules the dispersion force could dominate
To calculate the molar/molecular mass use the number below the symbol and then add up the numbers of each atom. Include 1 decimal place and the units g/mol
Lesson 9: Solubility of Molecular Substances
Glossary :
Solute = a substance like sugar
Solvent = a liquid like water
Solubility = If the solute dissolves in the solvent
Miscible = when 2 liquids are fully mixed in any proportion
Immiscible = when 2 liquids are never fully mixed in any proportion
The “Rule of Thumb” for predicting solubility or miscible vs immiscible is “like dissolves in like”. Polar dissolves in polar. Non polar dissolves in non polar.
The interactions between different polarity molecules would be weaker than the intermolecular bonding within the polar molecule. Therefore the bonds in the molecule would stay together and not interact with other molecules.