lecture 14: microtubules, MAPs and motors

Proteins can exist by themselves, bind to other proteins as dimers etc but the property of making chains is rare and is utilized by the cell for organisation, organisation and movement as well as cell division. these processes requires force. There is a network of polymers that enables transport and gives organization. Cells themselves also have to migrate a well as proteins eg in infection or for wound closure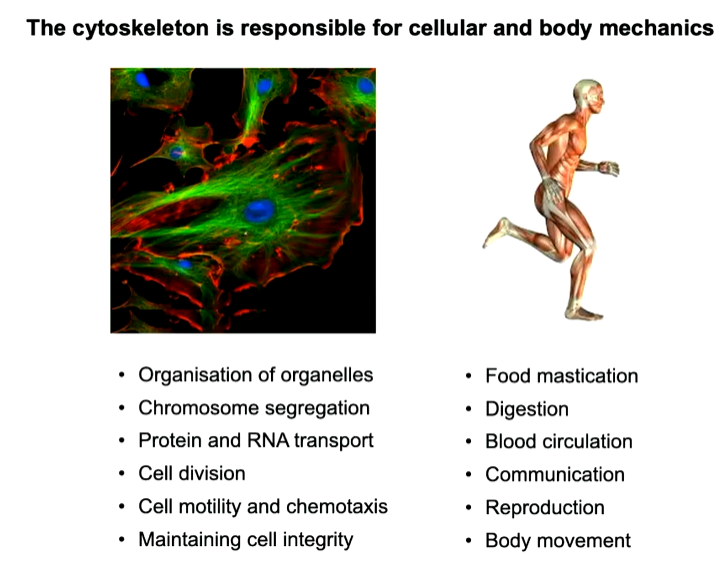
Introduction to the three major cytoskeletal systems
The cytoskeleton looks like this stained for the three major systems involved: 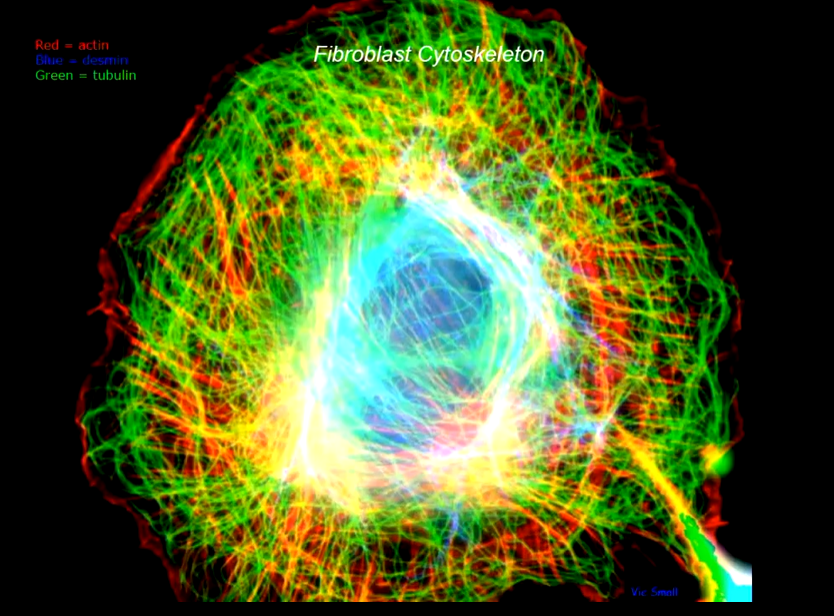
They can coexist in the same cell however not in the same space. At an atomic level: 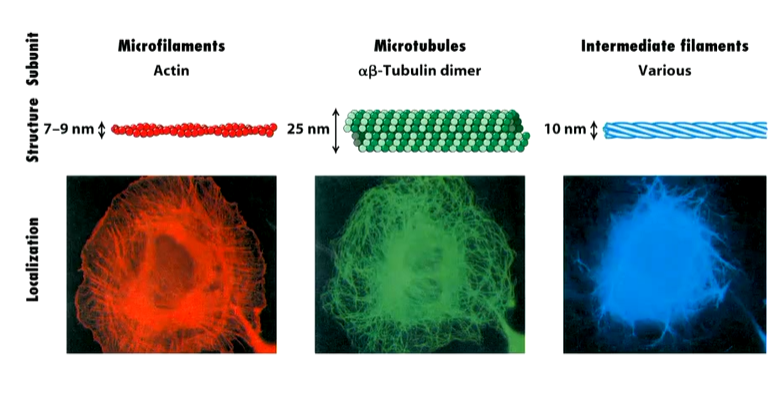
The thinnest of them is microfilaments of double helices of poly actin molecules which can be very long. Microtubules are made up of alpha and beta tubulins and are the widest with a hole through the middle making a tube. Intermediate filaments are intermediate in length and width. They are made up of various different proteins. To b able to make chains of cells is a rare property
The polymers also have different properties; 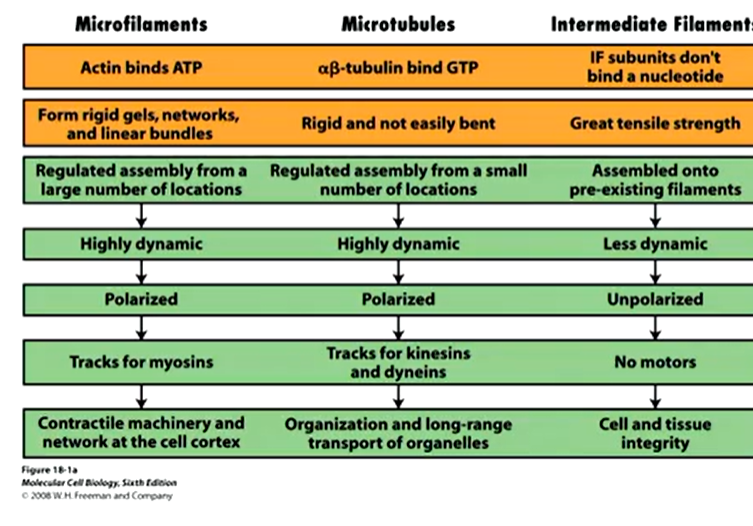
Microfilaments and microtubules require a fuel source to grow or shrink in order to be dynamic. Intermediate filaments don’t require this and resis force which he other two generate force.
Microtubules
This are the widest of the three and dynamic. Each microtubule in the network in the cell grows from a focus at the nuclear propriety. It is a barrel structure or tube made up of protofilaments which are made up of repetitive arrays of alpha and beta tubulin dimers that bind GTP.
The beta and alpha dimers look similar and make up protofilaments - 13 in a microtubule that makes a tube. It is stable as each dimer has a binding partner above and below as well as on the next protofilament either side of it. 
These structures are also dynamic so need to have a fuel source to build them - this comes from GTP for microtubules. Beta and alpha tubulins are globular proteins with clefts where it can bind. In B, the GTP undergoes hydrolysis but in a it stays as it is. If you are making a protofilament, it has polarity which is important as the tubes themselves have polarity - important for motor proteins as it gives directionality. As well as this, there are 13 protofilaments in a row with a lumen in the centre 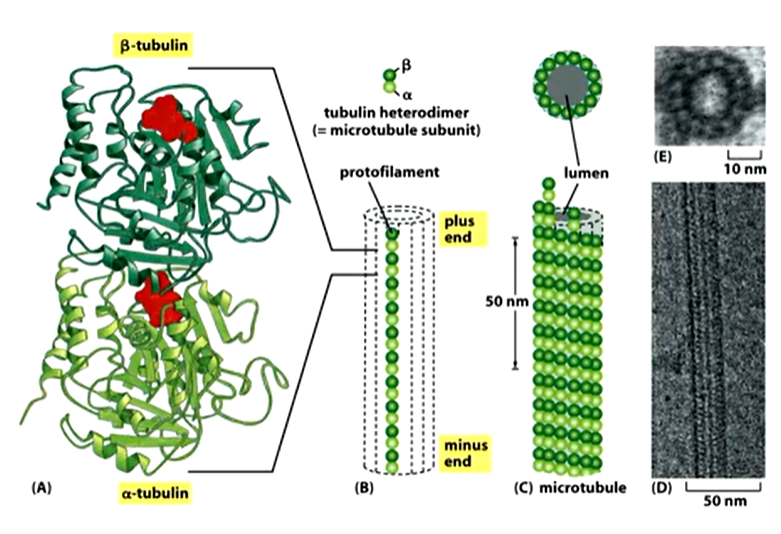
The b tubulin binds to an existing protofilament. In its GTP bound form, it will add on in solution to the plus end and in a weaker sense at the minus end. When these dimers are added, the GTP undergoes hydrolysis in the body of the tubulin. In this state, it won’t be utilized to bind onto a microtubule. 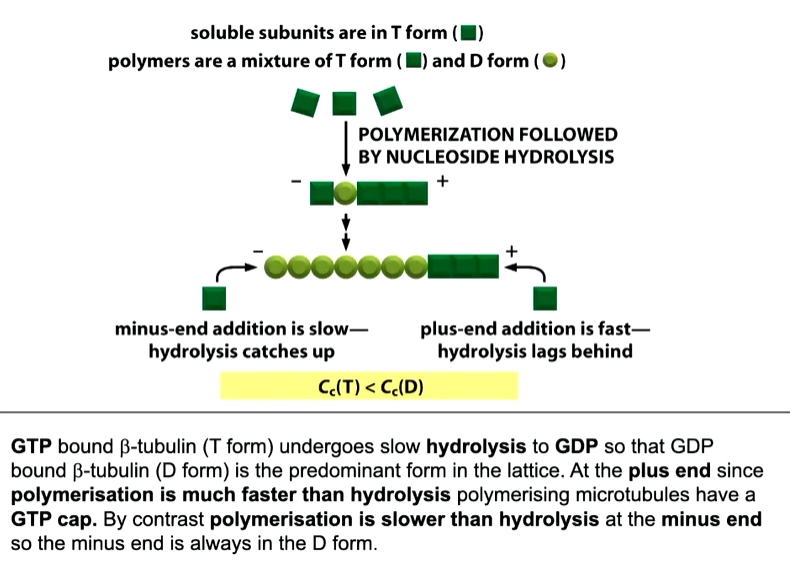
At the plus end - dynamic instability:
If you lose the ability to add new b tubulin dimers, those molecules at the + end will undergo hydrolysis and leave a GDP formed b tubulin. This tiggers a catastrophe event where growth stops and the microtubule depolymerisation until the depolymerising + end undergoes a rescue event where new GTP b dimers bind onto the end. 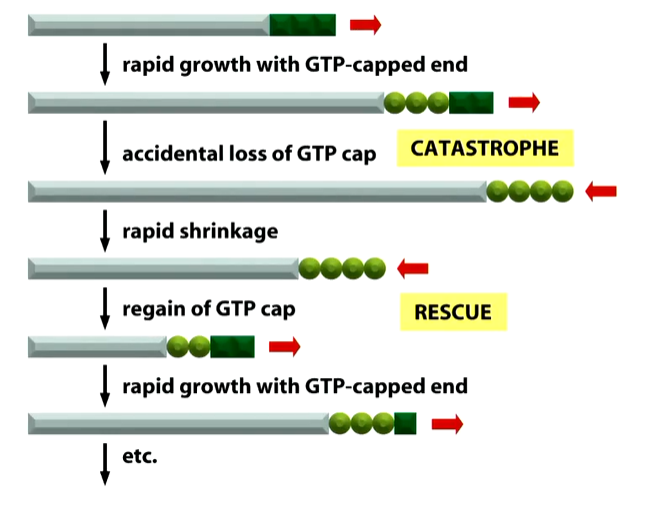
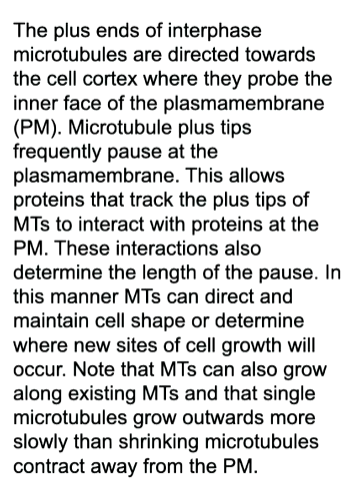
This is a mechanism for the cell to feel how big and what the shape of the cell is. The minus ends come from the centrosomes and are not dynamic.
The shape at the end of a microtubule are different when growing and shrinking. When it is growing and has GTP tubulin dimers at the end, it has a straight end but shrinking filaments have curved or frayed ends as there is GDP b tubulin at the + end causing rapid depolarisation. These are chopped up into individual a b dimers to be reused
An a b tubulin dimer in its GTp form, the angle is at a 5 degree shift in the axis however, when the GTP undergoes hydrolysis there is a conformational shift where there is now a 12 degree shift which causes the bent shape in rapid shrinkage 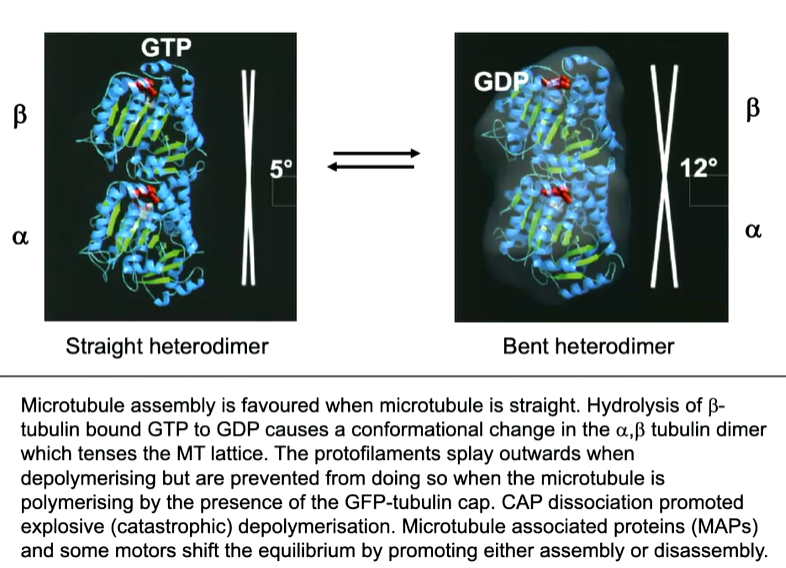
This is why when MTs are growing, they have a straight end byt when are shrinking, they have a curled end which makes it unstable. This can be utilised in order to transport at the end of a growing MT and release it when the MT shrinks. A series of 3 proteins one of which is called EB1 (end binding protein 1) and does not bind to GDP but does to the GTP cap at the end of the MT. 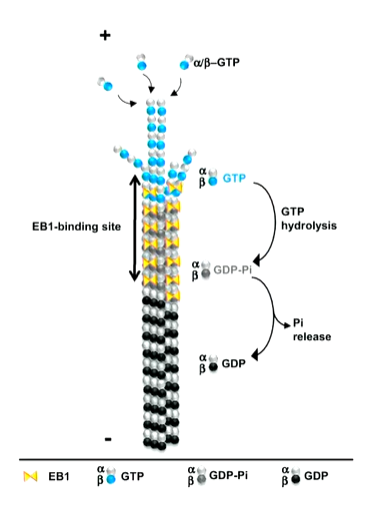
In this way, it is a marker for the growing + end. It HAS A BINDING SITE ON IT THAT ALLOWS OTHER PROTEINS SO THAT THEY CAN BE DEPOSITED WHEN THE MT UNDERGOES DISASSEMBLY.
Some drugs can cause MT instability and disassembly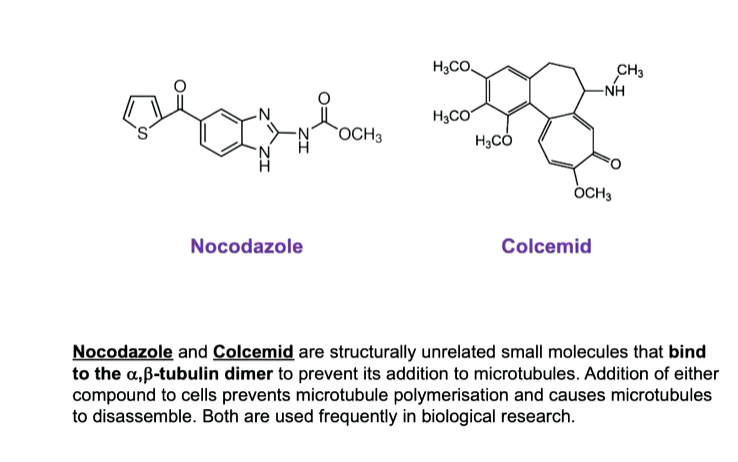
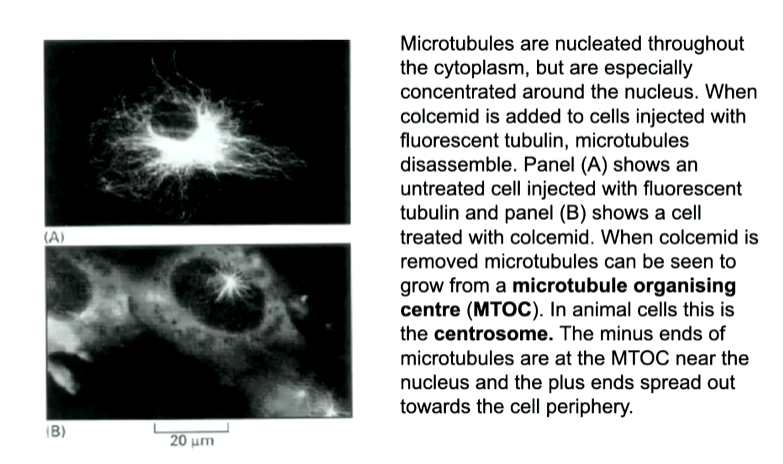
The point at where the MTs ar nucleasted are called an MTOC and is a way to keep the minus ends in a singular place. The major one in the cell is known as a centrosome.
centrosomes have centrioles in them surrounded by a pericentrol matrix in which minus ends are buried. Ring complexes called gamma tubulin ring complexes are where the MTs are organised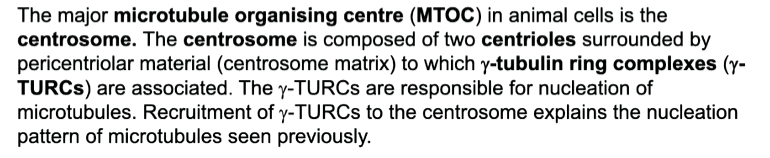
If the MTs are removed, you can see nucleating sites at the ring complexes. They have gamma tubulin that is a variant of a b tubulin that cannot make long polymers but can make one rng of 13 proteins. This nucleated the first addition of a b tubulin. Accessory proteins are added that allows the complex to be buried and bind to the centrosome 
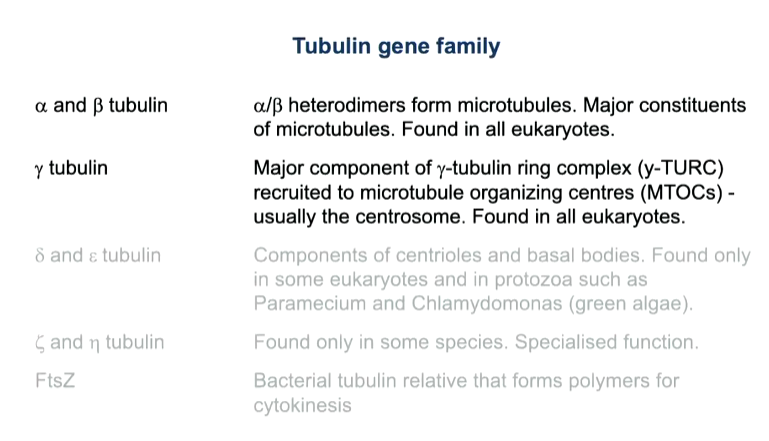
These microtubules are used for the organisation of organelles as well as protein and RNA transport as well as in mitosis for chromosomes segregation. An active transport system is required as it is a long way to travel - motor proteins that can move vesicles to the plus (kinesins) or minus ends (dynines) that use ATP as a fuel source. They organise and transport vesicles and chromosomes
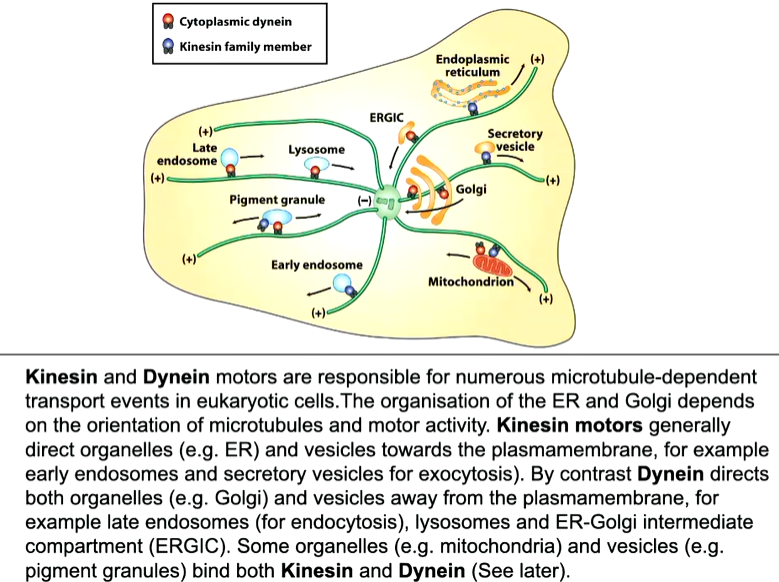
Golgi is closer to the nucleus than the ER as they are bound to different motors.

Kinesin
kinesin 1 dimers have heads that bind to the MT and walk along it with the other end bound to cargo - vesicle. In some cases the cargo can be other kinesin so MTs can slide along each other. 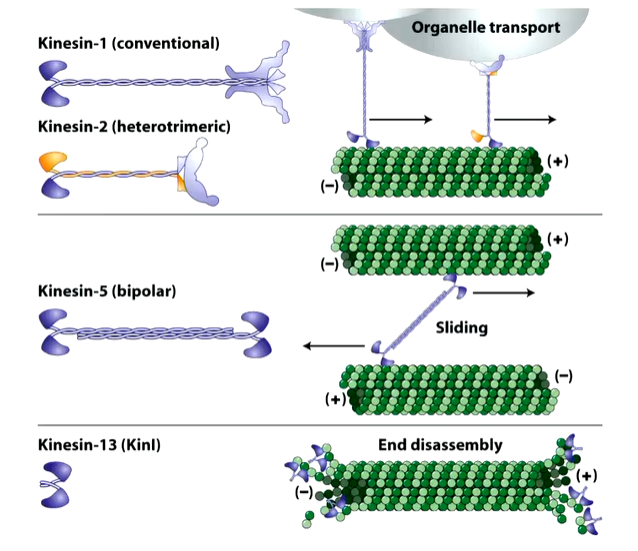
The head region is where the ADP/ATP is bound with a light chain at the other end that binds the cargo 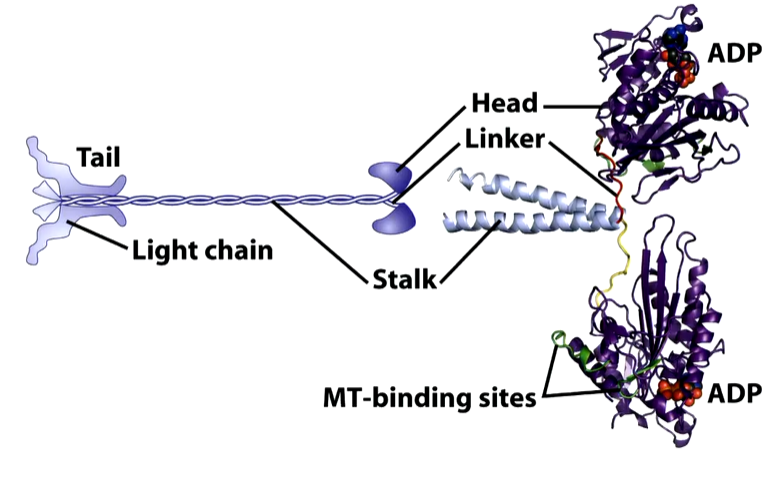
There are two identical heads that have a catalytic core and neck linkers. There are two identical motor heads. In solution, kinesin heads bind ADP and move randomly. When one kinesin head encounters a MT, it binds tightly and the ADP is ejected. ATP then enters the binding site. This exchange causes the neck linker to zip onto the catalytic core that throws the second head forward, The original head that bound hydrolysis the ATP and so has ADP bound. The head that was thrown forward now binds to the MT and the process is repeated. 
This uses 1 molecule of ATP per step.
Dynenin
This has associated proteins called dynactin complexes that allows attachment to the cargo as well as a second MT binding site. These move faster than kinesin and lose association with the MT but as you are attached, teh legs will go back down again and continue movement to the minus end. 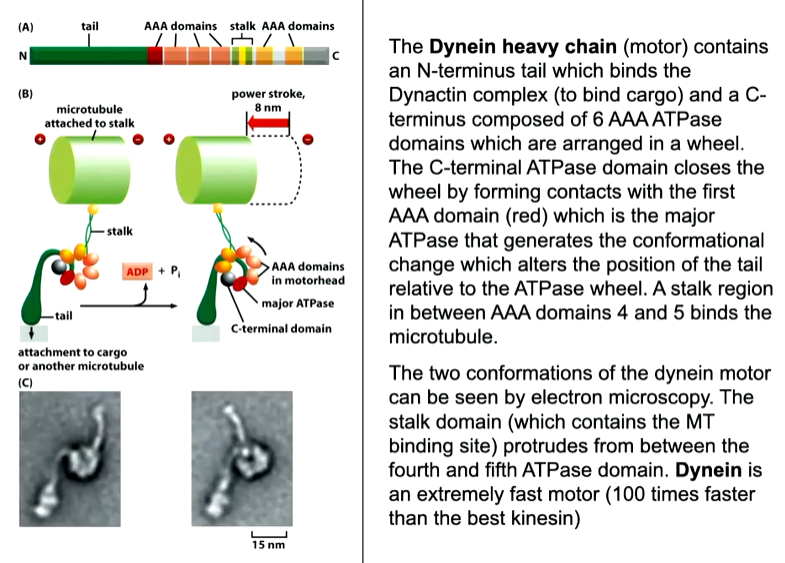
Motor proteins also have the ability to go around each other so that vesicles can go past each other.
Bi direction organelle or vesicle transport
Pigment granules and mitochondria are associated with both motor proteins
Pigment granules in melanosomes are found close to the minus end however, if fish get scared there is a hormonal signal that changes the activity of the motor proteins and they move towards the plus end. This will change the colour of the cell or fish 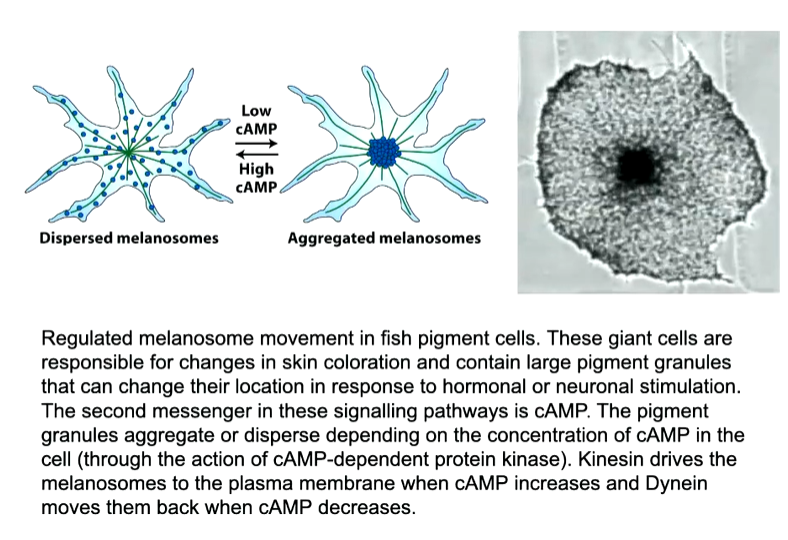
In neurons, to transport synaptic vesicles, there are MTs along the axon that also have polarity where the plus end is towards the synaptic terminal. Vesicles can move up and down the axon - the move in both directions as the vesicle or organelle is associated with both dynein and kinesin. This means the vesicle can deliver the neuropeptide at the synaptic terminal and then return to pick up more.