The Periodic Table
Periodicity: refers to trends in properties of elements across a period and down a group.
The columns of the table are called groups, and the rows are called periods.
The periodic table is organised by increasing atomic number. Elements in the same group (column) share the same number of valence electrons, leading to similar chemical properties.
Group 1: Alkali metals – soft, highly reactive, low melting points
Group 2: Alkaline earth metals – harder, less reactive
Groups 3–12: Transition metals – d-block with unique properties
Groups 13–18: Mix of non-metals, metalloids, halogens (Group 17), and noble gases (Group 18)
Group 17 (halogens) are very reactive non-metals needing one electron to complete their octet.
Group 18 (noble gases) are inert due to a full valence shell.
Lanthanides and actinides (f-block) are usually displayed separately at the bottom.
Metallic elements are found on the left-hand side of the table in the s block, in the central d block and in the island of the f-block. A small number of metals, like aluminium and lead, are also found on the left of the p-block.
Periods (rows) indicate the number of electron shells.
The periodic table is also divided into blocks based on the type of orbital being filled:
s-block: Groups 1–2
d-block: Transition metals
p-block: Groups 13–18
f-block: Lanthanides and actinides
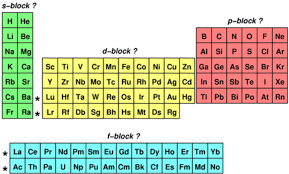
Elements whose valence electrons occupy an s sublevel make up the s block, which includes the alkali metals in group 1. Elements with valence electrons in p orbitals make up the p block, which includes halogens in group 17 and the noble gases in group 18. the d block and f block are similarly made up of elements with the outer electrons in d and f orbitals. the d block includes transition metals.
Properties
The effective nuclear charge experienced by an atom’s outer electrons increases across a period but remains approximately the same down a group.
Atomic radius decreases across a period due to increasing nuclear charge pulling electrons in.
Metals vs Non-metals
Metals (left side): lose electrons to form cations
Non-metals (right side): gain electrons to form anions
Bonding behavior is largely based on the drive to achieve a complete outer shell (octet)
Ionization energy is the energy needed to remove an electron. It increases across a period (stronger nuclear attraction) and decreases down a group (larger atoms, electrons farther from the nucleus).
Successive ionization energies rise significantly when removing inner shell electrons.
Ionization energy helps identify electron configurations and shell structure.
Sharp jumps in ionization energy indicate a new, more tightly bound shell is being accessed.
⚡Electron Affinity (EA)
Electron affinity: energy change when one mole of electron is added to one mole of gaseous atoms.
First EA is usually negative (exothermic) – energy is released
For metals: EA is small (less tendency to gain electrons)
Further electron additions (e.g., second EA) can be positive (endothermic) due to repulsion
Across a period: EA becomes more negative → atoms smaller, stronger attraction
Down a group: EA becomes less negative → added electrons further from nucleus
Electron affinity decreases down a group and increases across a period
💎 Electronegativity (EN)
Electronegativity: an atom’s ability to attract electrons in a covalent bond
Scale: 0 (least EN) to 4 (most EN, e.g., fluorine)
Across a period: EN increases (nucleus pulls shared electrons more strongly)
Down a group: EN decreases (increased shielding, electrons farther from nucleus)
Example: In H₂O, oxygen is more electronegative than hydrogen, attracting the bonding electrons closer.
🧾 Final Summary & Takeaways
Elements in the same group: same number of valence electrons
Elements in the same period: same number of shells
Trends across a period:
Atomic size decreases
Ionization energy, electron affinity, and electronegativity increase
Trends down a group:
Atomic size increases
Ionization energy and electronegativity decrease
Electron affinity becomes less negative
Metals, Non-Metals, and Metalloids
Metals have low electronegativity and ionization energy; they lose electrons and form metallic bonds.
Non-metals have high electronegativity and ionization energy; they gain/share electrons forming covalent bonds.
Metalloids (e.g., silicon, arsenic) have properties between metals and non-metals and are semiconductors.
Types of Bonding
Ionic bonds form when ΔEN > 1.8.
Covalent bonds form when ΔEN < 1.8, and polar covalent bonds fall between 0.5 and 1.8 ΔEN
BeCl₂ is covalent despite being a metal compound due to its low ΔEN.
Transition Metals
Found in the d-block, have partially filled d-orbitals or form ions with them
Form colored complexes with polar covalent coordinate bonds.
Zinc and scandium are not considered true transition metals due to lack of partially filled d-subshells.
Group 1 – Alkali Metals
Soft, low melting point metals that become more reactive down the group due to decreasing ionization energy
Reactivity example: potassium reacts more violently with water than sodium
Lower melting points down the group due to weaker metallic bonding
Group 17 – Halogens
Non-metals, exist as diatomic molecules, and have increasing boiling points down the group.
Reactivity decreases down the group for reduction reactions.
Dispersion forces increase with size, explaining phase changes: F₂ (gas), Cl₂ (gas), Br₂ (liquid), I₂ (solid).
Period 3 – Trends Across a Period
Atomic radius decreases, ionization energy and electron affinity increase left to right.
Metallic bonding (Na → Al) becomes stronger, increasing melting points
Si forms a giant covalent structure, making it brittle with high melting point
Non-metals (P, S, Cl, Ar) form simple molecules with weak intermolecular forces and low melting points
Oxides Across Periods 2 & 3
Oxides range from basic (e.g., Na₂O) to amphoteric (e.g., Al₂O₃) to acidic (e.g., SO₂, P₄O₁₀) across the period
Oxide character becomes more acidic across a period, and acidic oxides produce low pH solutions