Quater 3 - Periodic Table- TRENDS
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY). Click Here
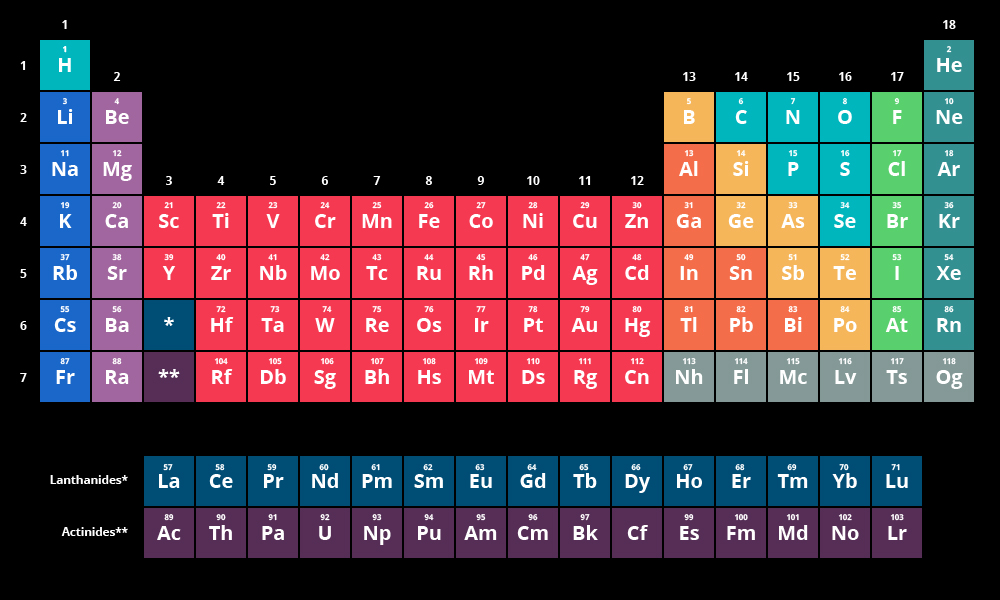
Periodic Table Basics:
- Ordered by Atomic Number (# of Protons)
- Columns are groups (Tend to have the same valence electrons)
- Rows are periods (Same amount of orbitals)
- Main Groups: (Divided by staircase on the right hand side. In yellow in the picture above)
- Metals- On the left side of the staircase.
- Reactive
- Ductile and Malleable
- Usually good conductors
- Usually solid at room temperature
- Nonmetals- On the right of the staircase.
- Brittle (Will break easily)
- Not always solid at room temperature
- Some of them are diatomic (Exist in pairs of atoms).
- Do not conduct electricity.
- Metalloids- Touching the staircase. (Yellow elements above)
- Can act like either a metal or a nonmetal depending on the conditions.
Other Groups: (Location on picture in parentheses)
- Alkaline: (Dark blue on left)
- Group 1 (1 valence electron)
- Very reactive with water
- Very soft
- Alkaline Earth: (Light purple on left)
- Group 2 (2 valence electrons)
- Also reactive with water
- Also soft
- Transition Metals: (Red in the middle)
- Groups 3-12 (Random amounts of valence electrons)
- Traditional metals like Copper
- Good conductors
- Hard
- Nonmetals: (Turquoise next to the yellow staircase)
- Groups 13-16 (3-6 valence electrons)
- Same characteristics as above for nonmetals
- Can be named more specifically by the element at the top of the group
- Halogens: (Lime green on the far right)
- Group 17 (7 valence electrons)
- Gasses
- Diatomic
- Noble Gasses: (Dark turquoise on the right end)
- Group 18 (8 valence electrons)
- Stable (Non-reactive)
- Gasses
Periodic Trends:
- Atomic Radius:
- Radius of an atom. Distance from the nucleus to its farthest orbital
- Increases from Right to left, and top to bottom on the table.
- Francinium (Fr) has the largest Atomic Radius.
- Electronegativity:
- Attraction of an atom to electrons
- Atoms will even attract its own electrons, pulling its orbitals farther in.
- Decreseres Atomic Radius
- Increases from left to right, and bottom to top on the table.
- Does not affect Group 18
- Categorized by a number 1-4 (4 being the strongest attraction)
- Fluorine (F) has the most attraction (4)
- Ionization energy:
- The amount of energy for an atom to take another atom’s electrons. To become an Ion.
- Atoms will attract not just it’s own electrons, but other atom’s electrons.
- Most atoms want 8 valence electrons so that's what they want to pull.
- Increases from left to right, and bottom to top on the table.
- Directly related to Electronegativity.
- Also does not affect group 18.
- Fluorine(F) is as
- Elements on the far left just easily give up electrons, rather than taking them.