22.1 Lattice enthalpy
Solid ionic compounds tend to be very stable - their stability arises from the strength of the ionic bonds, electrostatic attractions between oppositely-charged ions in the ionic lattice structure. This creates a substantial energy barrier that must be overcome to break down the lattice, reflected in the high melting points of ionic bonding in giant ionic lattice.
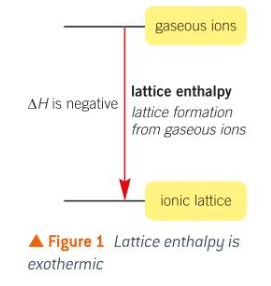
Lattice enthalpy - is the enthalpy change that accompanies the formation of one mole of an ionic compounds from its gaseous ions under standard conditions.
 It involves the ionic bond formation from separate gaseous ions. It is an exothermic change and the value for the enthalpy change will always be negative.
It involves the ionic bond formation from separate gaseous ions. It is an exothermic change and the value for the enthalpy change will always be negative.
Born-Haber cycle
Lattice enthalpy cannot be measured directly and must be calculated indirectly using known energy changes in an energy cycle.
Indirect determination of lattice enthalpy - requires a Born-Haber cycle.
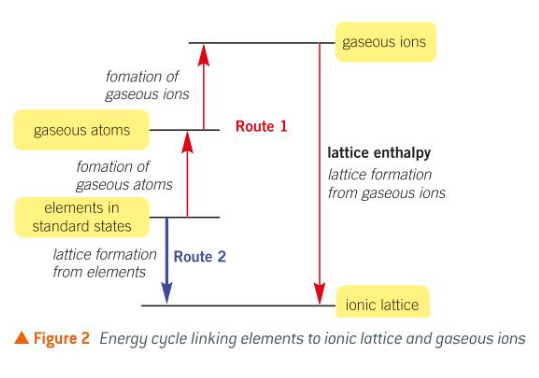
Route 1 - requires 3 different processes
Formation of gaseous atoms
changing the elements in their standard states into gaseous atoms
this change is endothermic as it involves bond breaking
Formation of gaseous ions
changing the gaseous atoms into positive and negative gaseous ions
overall this change is endothermic
Lattice formation
changing the gaseous ions into the solid ionic lattice
this is the lattice enthalpy and is exothermic
Route 2 - converts the elements in their standard states directly to the ionic lattice, there is just one enthalpy change, the enthalpy change of formation which is exothermic.
Key enthalpy changes
The standard enthalpy change of formation - is the enthalpy change that takes place when one mole of a compound is formed from its elements under standard conditions, with all the reactants and products in their standard states.
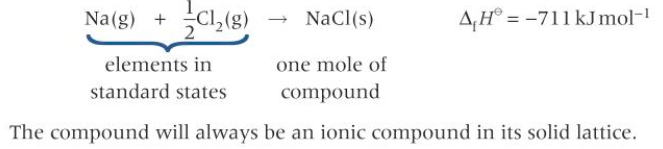 The standard enthalpy change of atomisation - is the enthalpy change that takes place for the formation of one mole of gaseous atoms from the element in its standard state under standard conditions.
The standard enthalpy change of atomisation - is the enthalpy change that takes place for the formation of one mole of gaseous atoms from the element in its standard state under standard conditions.
Always endothermic as bonds are broken to from gaseous atoms
When the element is a gas in its standard state, enthalpy change of atomisation is related to the bond enthalpy of the bond being broken.

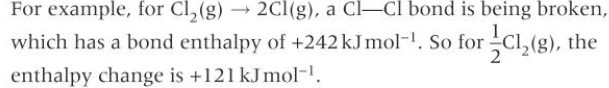
The first ionisation energy - is the enthalpy change required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ atoms.

Ionisation energies are endothermic because energy is required to overcome the attraction between a negative electron and the positive nucleus.
Electron affinity is the the opposite of ionisation energy
Electron affinity measures the energy to gain electrons
Ionisation energy measures the energy to lose electrons
The first electron affinity - is the enthalpy change that takes place when one electron is added to each atom in one mole of gaseous atoms to form one mole of gaseous 1- ions.

First electron affinities are exothermic because the electron being added is attracted to the nucleus.
Determination of lattice enthalpies - Born-Haber cycle
Successive electron affinities
When an anion has a greater charge than 1- such as O2- successive electron affinities are required.
Second electron affinities are endothermic, a second electron is being gained by a negative ion, which repels the electron away. Energy needs to be put in.