State of Matter and Mixtures
1.1 Three States of Matter
There are three states of matter: liquids, gases, and solids.
The existence of a chemical in all three states is typically dependant upon temperature and pressure.
At the melting and freezing points, respectively, states can shift from solid to liquid to gas to liquid and from gas to liquid to solid.
At the melting point, melting and freezing take place.
At the boiling point, condensation and boiling occur.
The characteristics of individual atoms differ from those of bulk materials.
A basic model can depict the three states of matter.
The particles in this model are represented by tiny, solid spheres.
Particle arrangement and movement
Particles classified as gases have the highest relative energy, solid particles have the lowest, and liquid particles fall in the middle. (All comparisons were made at room temperature).
The diagrams particles could be atoms, molecules, or ions, contingent upon the kind of material; examples include, metals, ionic compounds, simple molecules, and large molecules.
Interconversions
The strength of the forces between the particles determines how much energy is required to transition a state from solid to liquid and from liquid to gas.
An interconversion of state occurs when matter undergoes a change in state as a result of variations in pressure or temperature.
It is a physical alteration that involves adjustments to the forces that exist between the substance's particles; the substance's chemical characteristics and particles themselves stay unchanged.
Since no new substance is created during state conversions, physical alterations are generally simple to undo.
There are terminologies specifically used to characterise the interconversions:
Melting
Melting is when solid changes into a liquid.
Heat energy is needed for the operation, and this energy is converted to kinetic energy so that the particles can move.
It happens at a particular temperature that is particular to each pure solid and is referred to as the melting point.
Boiling
When a liquid changes into a gas, it's called boiling.
Heat is needed for this, as it creates gas bubbles beneath a liquid's surface that let liquid particles escape from both the liquid's surface and interior.
It happens at a particular temperature that is particular to every pure liquid and is referred to as the boiling point.
Freezing
When a liquid changes into a solid, it's called freezing.
Since this is the opposite of melting and happens at the same temperature as melting, a pure substance's melting and freezing points are the same.
For instance, at 0 °C, water freezes and melts.
It happens at a particular temperature that is particular to each pure substance and necessitates a large drop in temperature (or loss of thermal energy).
Evaporation
The change of a liquid into a gas is called evaporation.
Only in the liquid's surface, where high-energy particles can escape at low temperatures and below the liquid's boiling point, does evaporation take place.
A liquid can evaporate more quickly the larger its surface area and the warmer its surface.
A wide range of temperatures can cause evaporation, although heating will hasten the process since particles require energy to escape from the surface.
Condensation
The transition of a gas into a liquid, typically by cooling is called condensation.
Particles in a gas lose energy when it cools, and when they collide, they don't have the energy to bounce back and instead unite to form a liquid.
Sublimation
When a solid instantly transforms into a gas, it's called sublimation.
Only a few substances, like iodine or solid carbon dioxide, experience this.
The opposite reaction, known as desublimation or deposition, also occurs.
Predicting Physical State
It is possible to predict a substance's physical state under specific circumstances using a given set of data.
Typically, you are asked to predict a substance's physical state under particular conditions after being given information about its melting and boiling points.
Lower than the melting point temperatures:
The substance will be present in a solid condition.
When the temperature is below the boiling point but above the melting point:
The substance is going to be liquid.
Above the boiling point temperature:
The substance will be present in a gaseous form.
1.2 Pure Substances and Mixtures
Meaning of Pure
In chemistry, the term “pure" has a distinct meaning from its common usage.
For instance, stores offer containers of orange juice that are branded as "pure." The label indicates that there are no other ingredients—just orange juice.
But because it has several ingredients blended together, the juice isn't pure in a chemical sense.
In chemistry:
A pure material is made up of only one a single component or one compound
A mixture is made up of two or more distinct substances that aren't combined chemically.
Different types of chemical substances
There is only one type of atom in each element.
A compound is made up of two or more different types of atoms together.
A mixture is made up of two or more distinct components that aren't combined.
A mixture's constituent materials may be elements or compounds.
A few examples are displayed in the table:
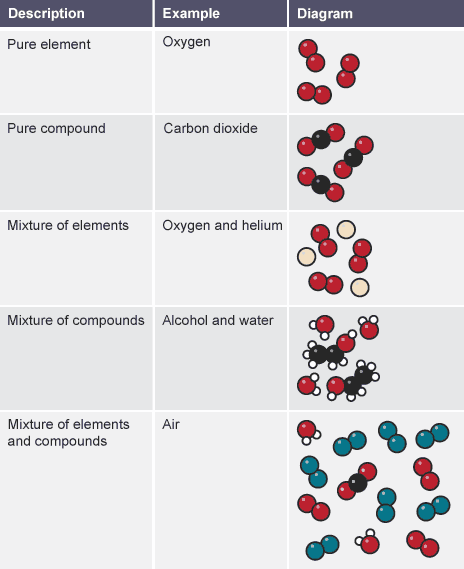
Differentiating between mixtures and pure substances
Pure materials possess a sharp melting point, however mixtures can melt at many different temperatures.
The easiest way to notice this difference is to measure the temperature of a hot liquid as it cools and freezes.
The cooling curve for a sample of the substance salol is displayed on the graph.
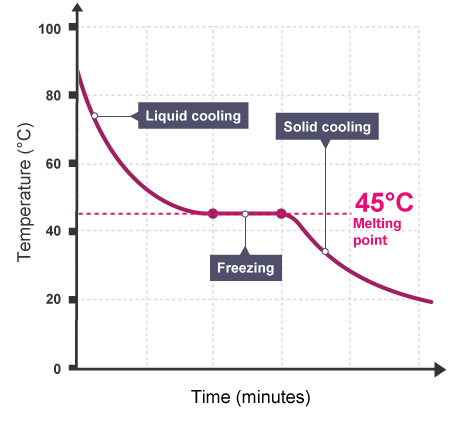 When a pure substance changes states, the temperature remains constant.
When a pure substance changes states, the temperature remains constant.
The graph's horizontal section indicates that the salol is pure because it has a sharp melting point.
When impure salol (a combination of salol and other materials) freezes, it will gradually lose volume at different temperatures.
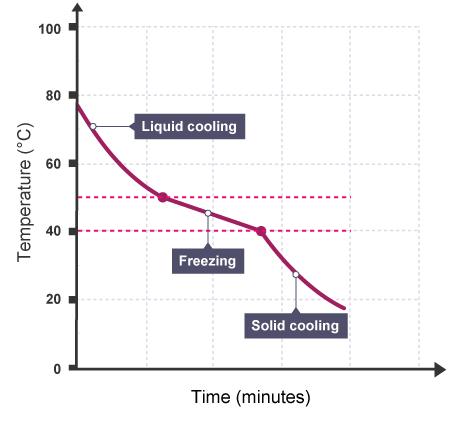 When an impure substance changes state, the temperature changes slightly.
When an impure substance changes state, the temperature changes slightly.
Crystallization and Filtration
Crystallization
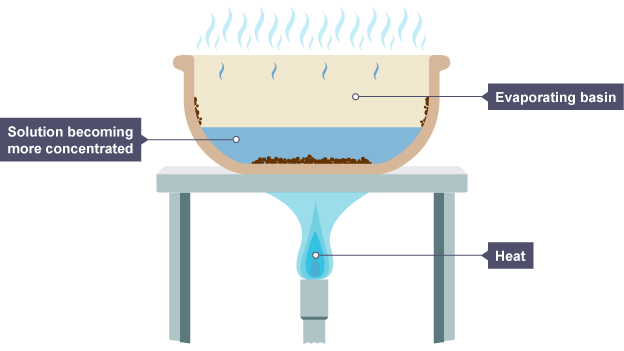
The process of crystallisation is used to turn a solution into solid crystals.
A more concentrated solution is left behind after some of the solvent evaporates when the solution warms up.
To produce big, uniformly formed crystals:
Place the mixture in an evaporating bowl.
By setting the evaporating basin over a bath of hot water, you can warm the solution.
Before the entire solvent has evaporated, turn off the heat.
Filtration
To extract an insoluble substance from a liquid, filtering is utilised.
It can be used to remove excess reactant from a solution or separate sand from a combination of sand and water.
Because the filter paper has tiny holes, or pores, in it, filtration is effective.
These are sufficiently big to allow tiny molecules to and dissolved ions through, but not the significantly bigger undissolved solid particles.
Distillation
Simple distillation
A solvent and a solution can be separated using simple distillation. It works well for making water out of a salt solution.
Because the dissolved solute is what makes simple distillation work boils at a significantly higher temperature than the solvent.
Solvent vapour from the solution evaporates when it is heated. The gas disperses, cools, and compacted.
As the amount of solvent in the remaining solution drops, the solute concentration in it increases
Fraction Distillation
Different liquids can be extracted from a combination of liquids using fractional distillation.
It works well for separating various substances, such as ethanol from a combination of ethanol and water components of crude oil
The reason fractional distillation works is that the boiling points of the various liquids vary. As soon as the mixture is heated:
Vapours ascend via a column that is cold at the top and hot at the bottom.
When vapours come into contact with a region of the column that is colder than their boiling point, they condense.
The liquid exits the column.
1.3 Paper chromatography
Mixtures of soluble materials are separated using paper chromatography.
These are frequently colourful materials like plant pigments, food colouring, inks, and dyes.
Chromatography is dependent upon two distinct 'phases':
the stationary phase, which in paper chromatography is relatively uniform, absorbent paper
the mobile phase is the solvent that passes through the paper, carrying different substances with it
In varying amounts, the various dissolved materials in a mixture are drawn to the two phases. They proceed through the paper at varying speeds as a result.
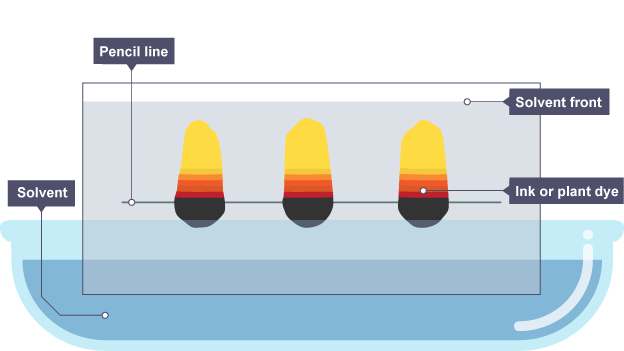
Interpreting a Chromatogram
A chromatogram is the result of chromatographic separation.
It is possible to discern between pure and impure substances using a paper chromatogram:
A single spot appears on the chromatogram for a pure material.
Two or more spots are produced by an impure substance.
By contrasting chemicals with known substances, a paper chromatogram can also be used to identify substances. If two chemicals are similar, they probably are if:
they both create an equal amount of spots that are the same colour.
the spots have the same Rf value and move the same distance up the paper.
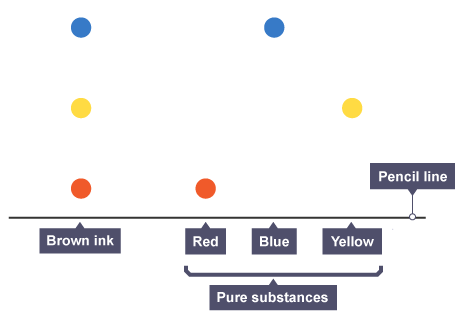
The red, blue, and yellow inks are mixed to create the brown ink in this chromatogram. This is as a result of the brown ink's dots having the same Rf value and heights as the reference inks.
Rf values
If unknown chemicals can be compared to a variety of reference materials, they can be identified using Rf values.
For a given substance, the Rf value is constant.
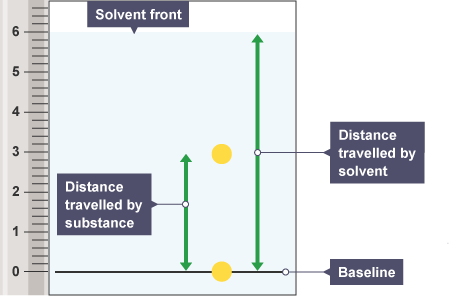
A spot's Rf value is determined by using:
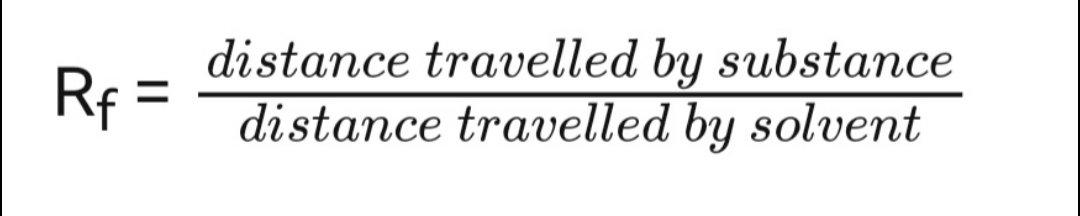
Rf values are always between 0 and 1, and they are expressed as rounded decimals.
When the Rf value is zero, it indicates that the material is not soluble in the solvent being employed, which keeps the spot on the starting line.
When the substance has migrated as far as the solvent front, as indicated by an Rf value of 1, it is strongly attracted to the mobile phase and not to the stationary phase, which is the paper.
1.4 Purifying Water
Groundwater and waste water need to be treated in order to make them safe for drinking.
Purifying fresh water
To make fresh water drinkable, sediments must be removed. These consist of big things like leaves and branches, as well as insoluble particles like dirt and dangerous microbes.
To deal with them, many techniques for separation and therapies are employed:
Large objects are eliminated by grid-based filtering.
Larger insoluble grit particles are removed using a coarse filter bed composed of clean sand and gravel.
In a sedimentation tank, smaller insoluble particles are clumped together and sink to the bottom with the addition of aluminium sulphate.
Very tiny insoluble particles are removed by a fine filter bed.
The addition of chlorine gas kills dangerous bacteria
Purifying seawater
Seawater is unfit for human consumption due to its high concentration of dissolved salt.
Nevertheless, seawater can be used to create pure water by elementary distillation.
After boiling the seawater, the water vapour is cooled and guided away. It condenses, removing the salt and forming clean water.
This method of producing drinking water is costly since it requires a lot of energy to heat the seawater.
Distillation yields water, though, which can be used in the lab to dissolve materials. There are no dissolved ions in it that could obstruct a chemical analysis.