Standard Cell Potentials
*These notes were taken from LG 5.4
LG:
file:///C:/Users/Valeska/Desktop/Valeska's%20School%20Files/PSHS%20Grade%2010/Chemistry%202/4th%20Quarter/Learning%20Guides/SLG%20CHEM2%20LG%205.4%20Standard%20Cell%20Potentials.pdf
Test your knowledge by answering the following:
1. For the reaction:
Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
a. Write the oxidation half-reaction.
b. Write the reduction half-reaction.
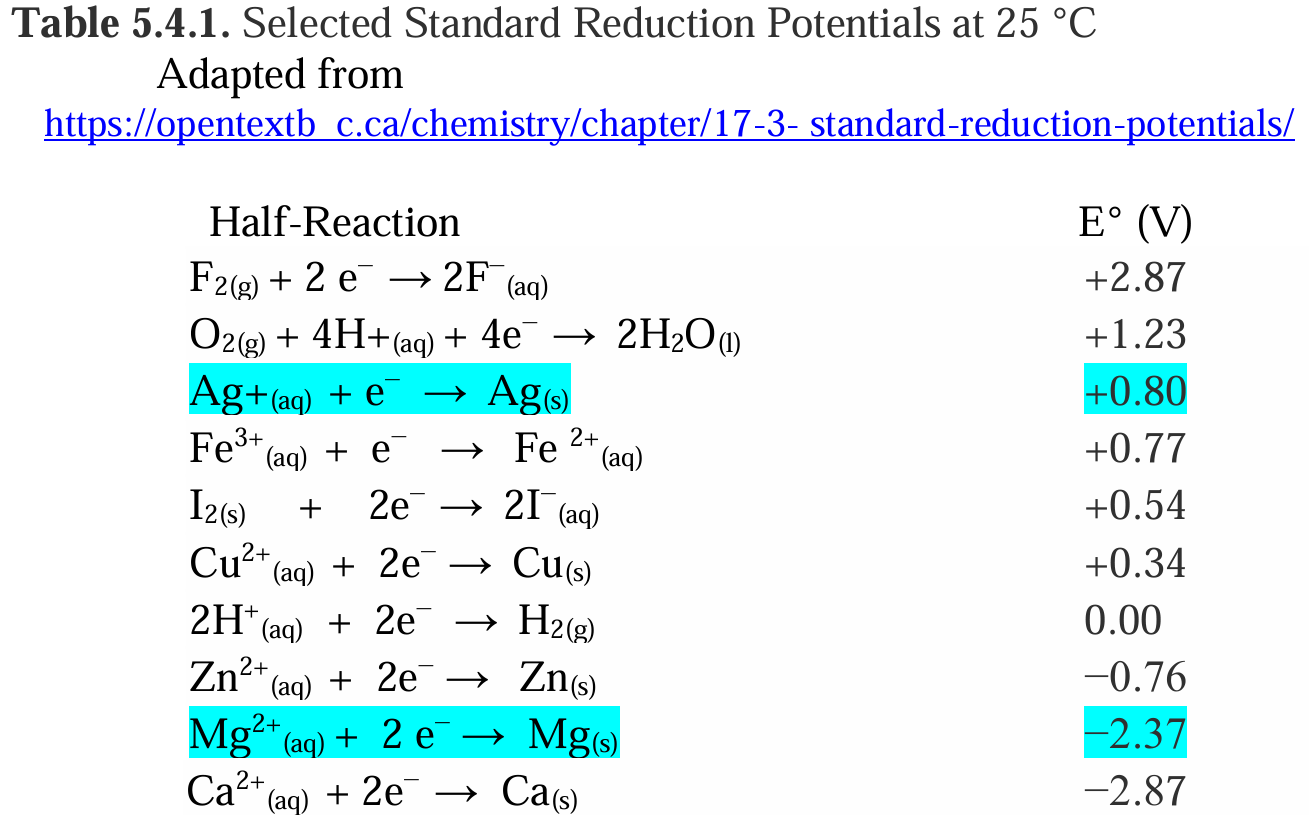
c. Use Table 5.4.1 to find each Eo half-cell.
d. Calculate the Eocell for the reaction.
e. Is the reaction spontaneous?
2. Write the balanced equation, calculate Eocell and state whether the reaction is spontaneous:
I2(g) + Fe2+(aq) → I–(aq) + Fe3+(aq)
Answer Key:
Let’s carefully solve each part of the electrochemistry questions using Table 5.4.1 from the image. We’ll use the formula for cell potential:
Ecell∘=Ecathode∘−Eanode∘E^\circ_{\text{cell}} = E^\circ_{\text{cathode}} - E^\circ_{\text{anode}}
🔹 1. For the reaction:
Cu(s)+2Ag(aq)+→Cu(aq)2++2Ag(s)\text{Cu}_{(s)} + 2Ag^+_{(aq)} \rightarrow \text{Cu}^{2+}_{(aq)} + 2Ag_{(s)}
a. Oxidation half-reaction (Cu loses electrons):
Cu(s)→Cu(aq)2++2e−\text{Cu}_{(s)} \rightarrow \text{Cu}^{2+}_{(aq)} + 2e^-
b. Reduction half-reaction (Ag⁺ gains electrons):
Ag(aq)++e−→Ag(s)\text{Ag}^+_{(aq)} + e^- \rightarrow \text{Ag}_{(s)}
(We multiply this by 2 to balance electrons.)
c. Standard reduction potentials (from table):
EAg+/Ag∘=+0.80 VE^\circ_{\text{Ag}^+/Ag} = +0.80 \, \text{V}
ECu2+/Cu∘=+0.34 VE^\circ_{\text{Cu}^{2+}/Cu} = +0.34 \, \text{V}
But Cu is oxidized, so we reverse the reaction, and use:
ECu/Cu2+∘=−0.34 VE^\circ_{\text{Cu/Cu}^{2+}} = -0.34 \, \text{V}
d. Calculate Ecell∘E^\circ_{\text{cell}}:
Ecell∘=Ecathode∘−Eanode∘=(+0.80 V)−(+0.34 V)=+0.46 VE^\circ_{\text{cell}} = E^\circ_{\text{cathode}} - E^\circ_{\text{anode}} = (+0.80\, \text{V}) - (+0.34\, \text{V}) = \boxed{+0.46\, \text{V}}
e. Is the reaction spontaneous?
✅ Yes. The cell potential is positive, so the reaction is spontaneous.
🔹 2. For the reaction:
I2(g)+Fe(aq)2+→I(aq)−+Fe(aq)3+I_2(g) + Fe^{2+}_{(aq)} \rightarrow I^-_{(aq)} + Fe^{3+}_{(aq)}
a. Identify half-reactions:
Reduction: Fe3++e−←Fe2+⇒Fe2+→Fe3++e−Fe^{3+} + e^- \leftarrow Fe^{2+} \Rightarrow \text{Fe}^{2+} \rightarrow \text{Fe}^{3+} + e^-
→ This is oxidation.Oxidation: I2+2e−→2I−I_2 + 2e^- \rightarrow 2I^-
→ This is reduction.
So:
Anode (oxidation): Fe2+→Fe3++e−Fe^{2+} \rightarrow Fe^{3+} + e^-
Cathode (reduction): I2+2e−→2I−I_2 + 2e^- \rightarrow 2I^-
We multiply the Fe reaction by 2 to balance electrons.
b. Balanced equation:
I2+2Fe2+→2I−+2Fe3+I_2 + 2Fe^{2+} \rightarrow 2I^- + 2Fe^{3+}
c. Standard reduction potentials (from table):
EI2/I−∘=+0.54 VE^\circ_{\text{I}_2/ I^-} = +0.54 \, \text{V}
EFe3+/Fe2+∘=+0.77 VE^\circ_{\text{Fe}^{3+}/Fe^{2+}} = +0.77 \, \text{V}
But Fe²⁺ is oxidized, so reverse its reduction potential:
EFe2+/Fe3+∘=−0.77 VE^\circ_{\text{Fe}^{2+}/Fe^{3+}} = -0.77\, \text{V}
d. Calculate Ecell∘E^\circ_{\text{cell}}:
Ecell∘=+0.54 V−(+0.77 V)=−0.23 VE^\circ_{\text{cell}} = +0.54\, \text{V} - (+0.77\, \text{V}) = \boxed{-0.23\, \text{V}}
e. Is the reaction spontaneous?
❌ No. The cell potential is negative, so the reaction is non-spontaneous under standard conditions.
Let me know if you want this compiled into a printable summary or table.