Lecture 8: Gluconeogenesis in Detail
Learning Outcomes:
Define the key steps involved in the conversion of 3-carbon intermediates to 6-carbon intermediates in gluconeogenesis
Review the steps involved in the release of glucose from cytosolic glucose 6-phosphate
Analyse the effects of the absence of the 2-OH group in glucose and generate rationales for the use of 2-deoxy derivatives in clinical diagnostics
Predict the flow of glycolysis and gluconeogenesis based on the relative activities of phosphofructokinase and fructose 1,6 bisphosphatase
Compare the regulators of phosphofructokinase and fructose 1,6 bisphosphatase
Outline the reasons to produce fructose 2,6 bisphosphate
Apply knowledge of the effects of fructose 2,6 bisphosphate to the regulation of PFK and F16BPase
Illustrate the dual identity of PFK/F26BPase through phosphorylation changes
Outline the consequences of changes in PFK/F26BPase activity
Compare the conversion of pyruvate to phosphoenolpyruvate in gluconeogenesis to the opposite process in glycolysis
Predict the effect of fatty acid oxidation on glucose fluxes as a consequence of its effects on pyruvate carboxylase
Evaluate the importance of anaplerosis of Krebs Cycle intermediates
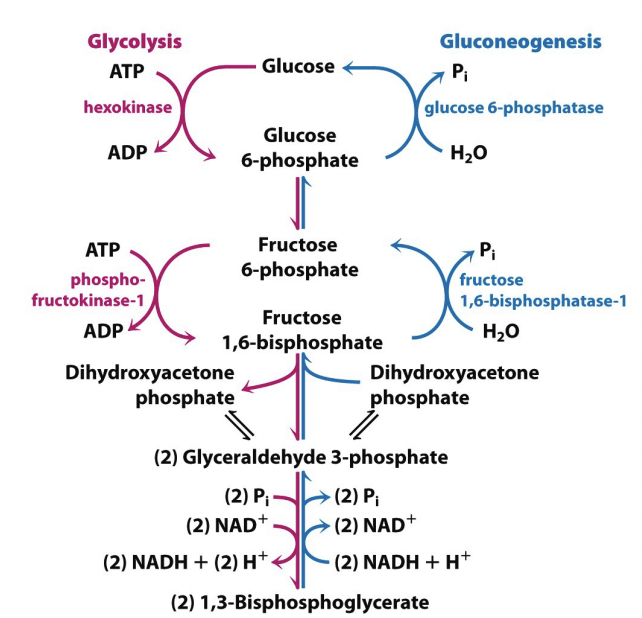
Getting from 3C to 6C:
Require phosphatase rather than kinase
Don’t get the ATP back that we invested – you do in glycolysis
Phosphatases are named by substrate
Fructose 1,6-biphosphatase
Glucose 6-phosphatase
Phosphatases remove phosphate groups
Double headed arrow indicate equilibrium reactions – don’t need enzymes to go back either way
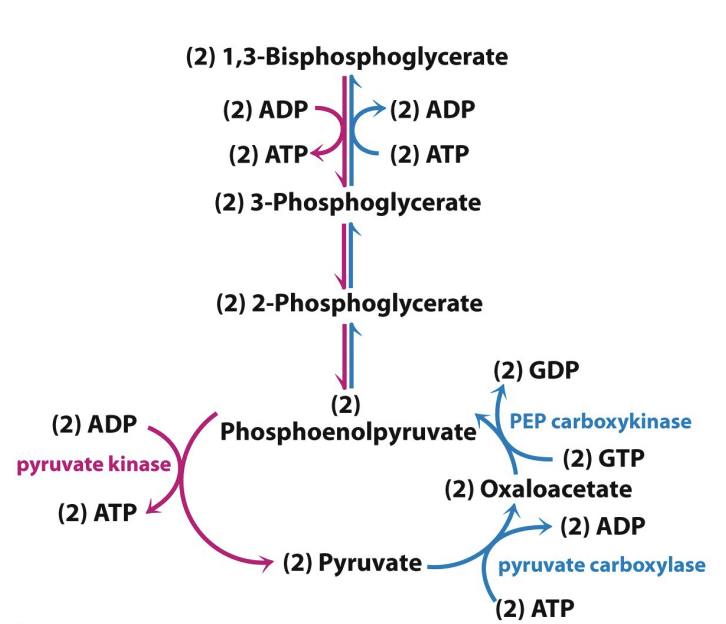
Using the ATP:
Need to bypass glycolytic energy releasing steps
Need two enzymes to convert pyruvate to phosphophenolpyruvate
Pyruvate carboxylase (PC) is mitochondrial
PEP carboxykinase (PEPCK) varies
Pyruvate → Oxaloacetate (via PC)
Oxaloacetate → phosphophenolpyruvate (via PEPCK)
Gluconeogenisis mostly happens in the liver, but these enzymes exist in other tissues as well
PC exists in most tissues
Pyruvate is a 3C molecule
Oxaloacetate is a 4C molecule
First carrier in the Krebs cycle with Acetyl-CoA
PEPCK uses GTP instead of ATP but it does the same thing – gives a phosphate
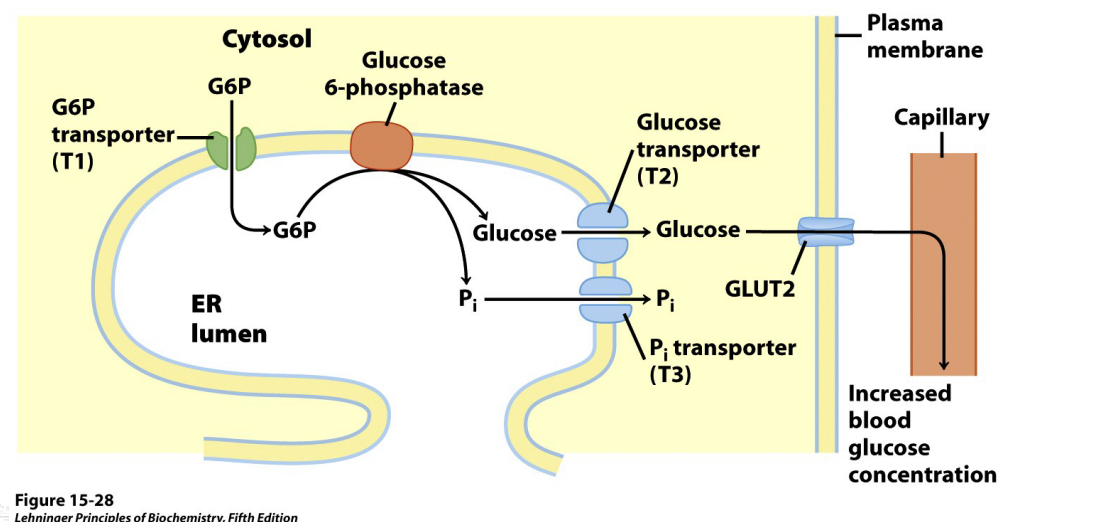
G6Pase System – Only in Liver:
In the liver, to get glucose 6-phosphate back to glucose is in the endoplasmic reticulum
Glucose can make its way out back into the blood stream via GLUT 2 transporters
GLUT2 allows equilibrium to be established
G6P transporter allows G6P into the ER
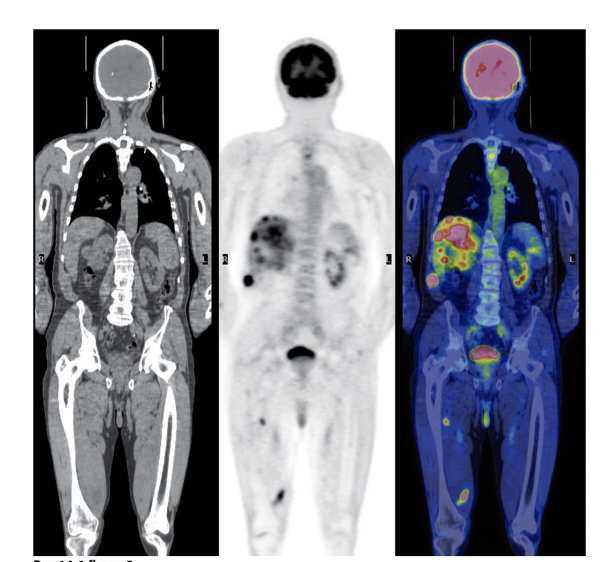
G6Pase is targeted in diagnostics:
To diagnose cancer
Glucose molecule with a radioactive fluorine
Cells will treat the 2-fluro-deoxyglucose like any other glucose and transport it into the cell
They will trap it by phosphorylating it with hexokinase
The molecule can’t continue journey down glycolysis to fructose-6-phosphate
It gets trapped and accumulates in the cell/cytoplasm
Cancer cells rely heavily on glucose to lactate metabolism – only getting 2 ATPs
Radio labelling the glucose can allow screening (full body PET Scan) – high concentration in cancer areas
Ignore brain (uses a lot of glucose) and bladder (getting rid of unusable glucose)
Reversing the PFK Step of Glycolysis:
Cells need to decide if they are going to break down or store glucose – complicated
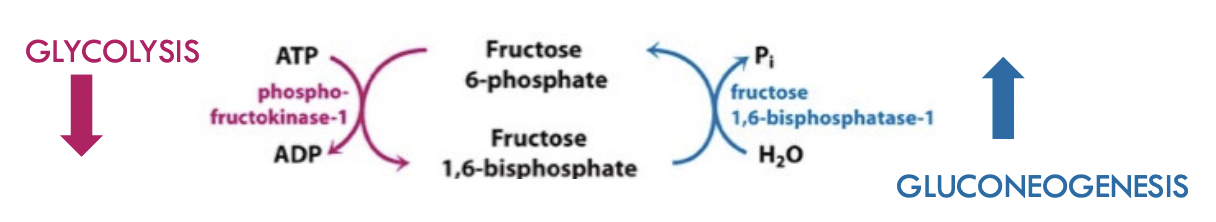
Cell needs to regulate if they use PFK-1 or FBPase-1
Controlled through allosteric regulators:
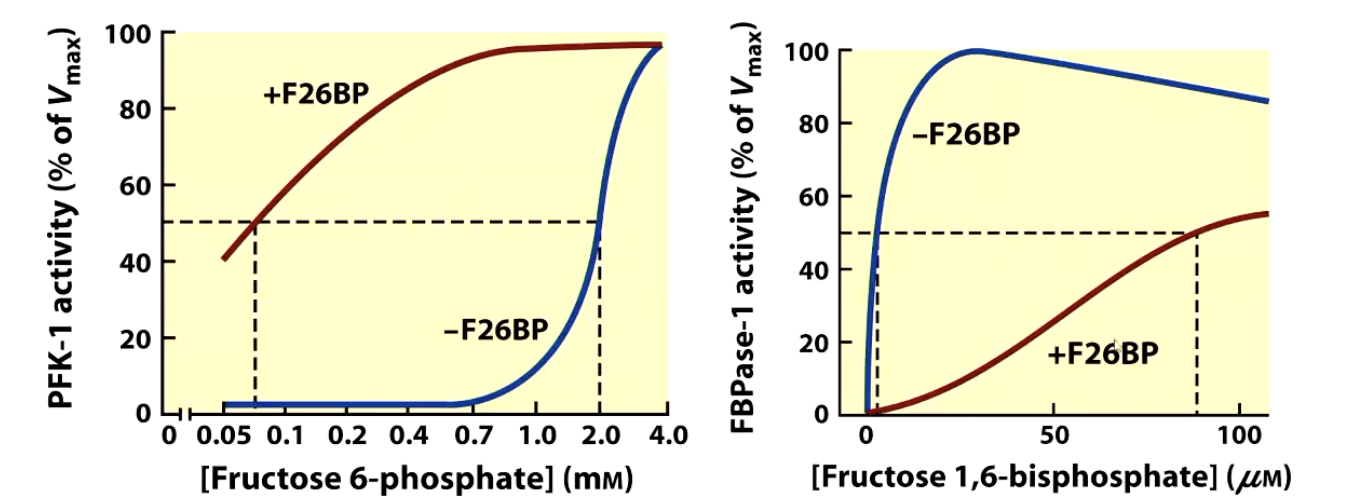
Increase ATP negatively regulates PFK-1 to down regulate glycolysis
Increase in ADP positively regulates PFK-1 to upregulate glycolysis
Increase in AMP can switch on gluconeogenisis and switch ofd PFK-1
Increase in citrate negatively regulates PFK-1to down regulate glycolysis
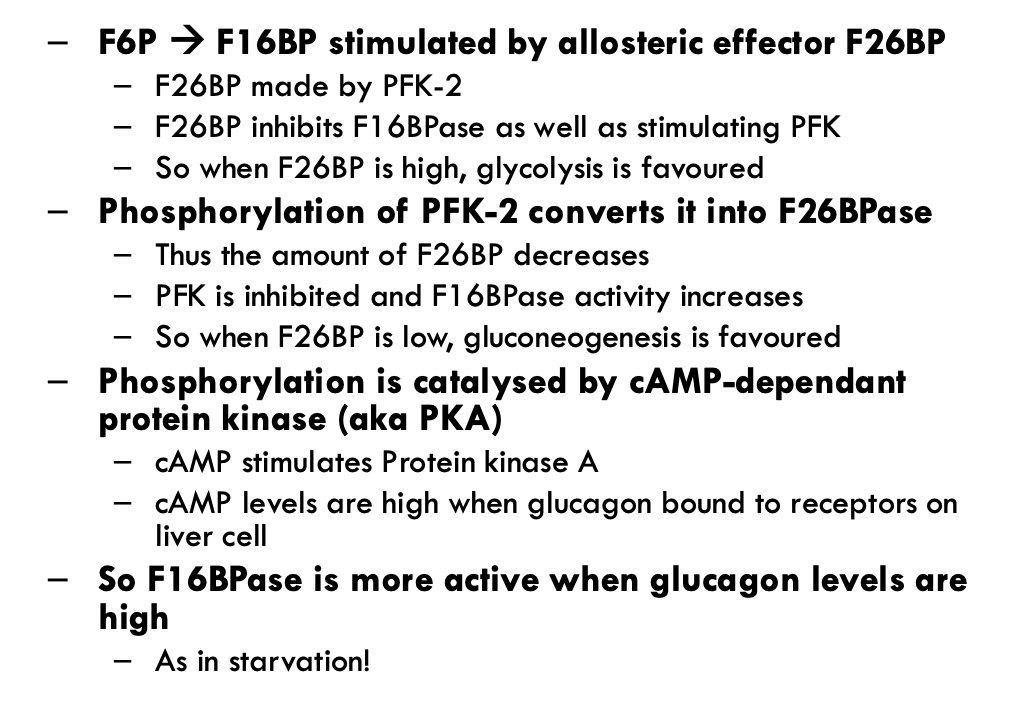
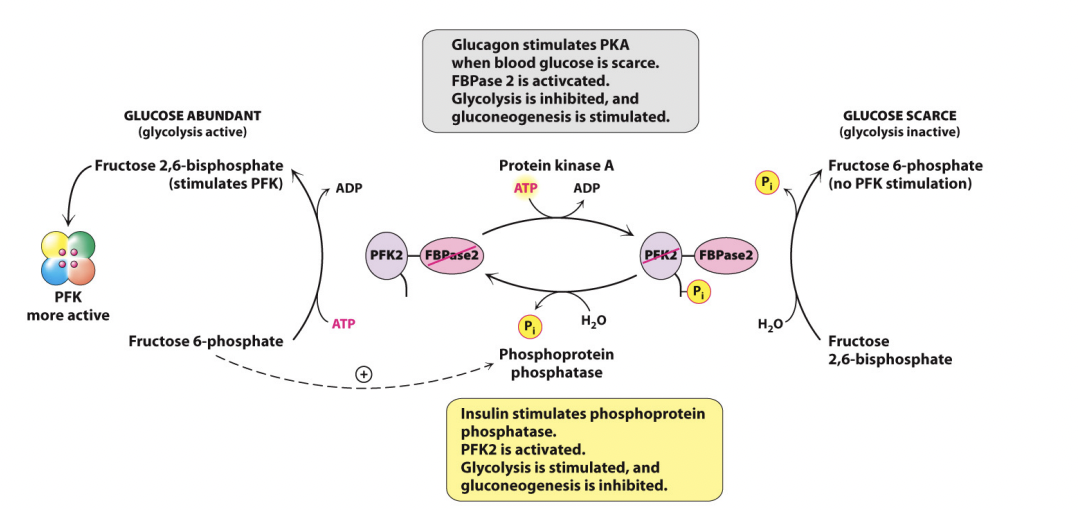
Completely Different Pathway to Glycolysis:
Fructose 6-phosphate to Fructose 2,6-bisphosphate using PFK-2
Opposite way using FBPase-2
Fructose 2,6-bisphosphate (F26BP) is a regulatory molecule and it can only get phosphorylated or dephosphorylated in the loop
Fructose 2,6-bisphosphate is only formed in small amounts but is more powerful in regulating glycolysis than anything also
Works at the same step using PFK-1 and FBPase-1
Fructose 6-phosphate to Fructose 1,6-biphosphate (and reverse for gluconeogenisis)
Adding F26BP to the factors that control whether a cell does glycolysis or gluconeogenisis
Increase in F26BP upregulates PFK-1and glycolysis
Increase in F26BP downregulates FBPase-1 and gluconeogenisis
The most powerful regulator
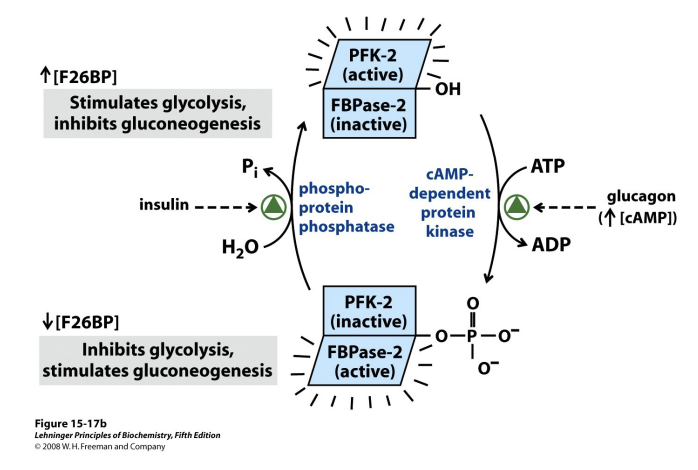
PFK-2 and FBPase-2 are the same enzyme
Swapping from one to another after reversible phosphorylation
Insulin and glucagon determine which parts of the enzyme is active
Interversion catalysed by Protein Kinase A (PKA)
Sensitive to cAMP
And therefore, glucagon/insulin
PKA can phosphorylate the enzyme in the presence of glucagon and activate the FBPase-2 which inhibits glycolysis and stimulates gluconeogenisis
PKA can activate the phosphatase and remove the phosphate group in the presence of insulin to stimulate glycolysis and inhibit gluconeogenisis
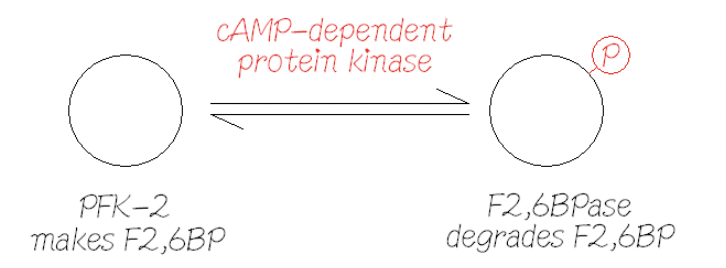
When F26BP is high, glycolysis is favours
When F26BP is low, gluconeogenisis is favoured
Summary:
Reversing the PEP to Pyruvate – Early Steps of Gluconeogenisis:
Requires 2 ATP consuming steps
Pyruvate carboxylase – stimulated by acetyl-CoA (fatty acid oxidation)
Want to create more glucose
Also creates oxaloacetate which can be used in the Krebs cycle
PEPCK (Pepcarboxykinase)
Stimulated by increased transcription/translation of the gene
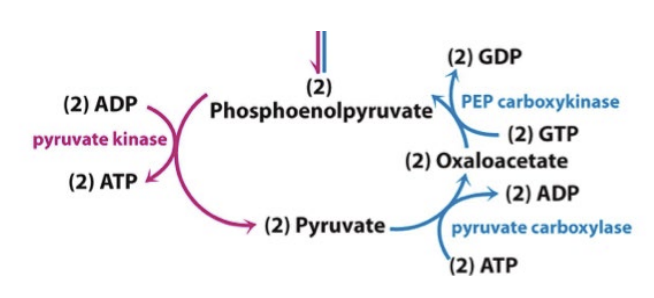
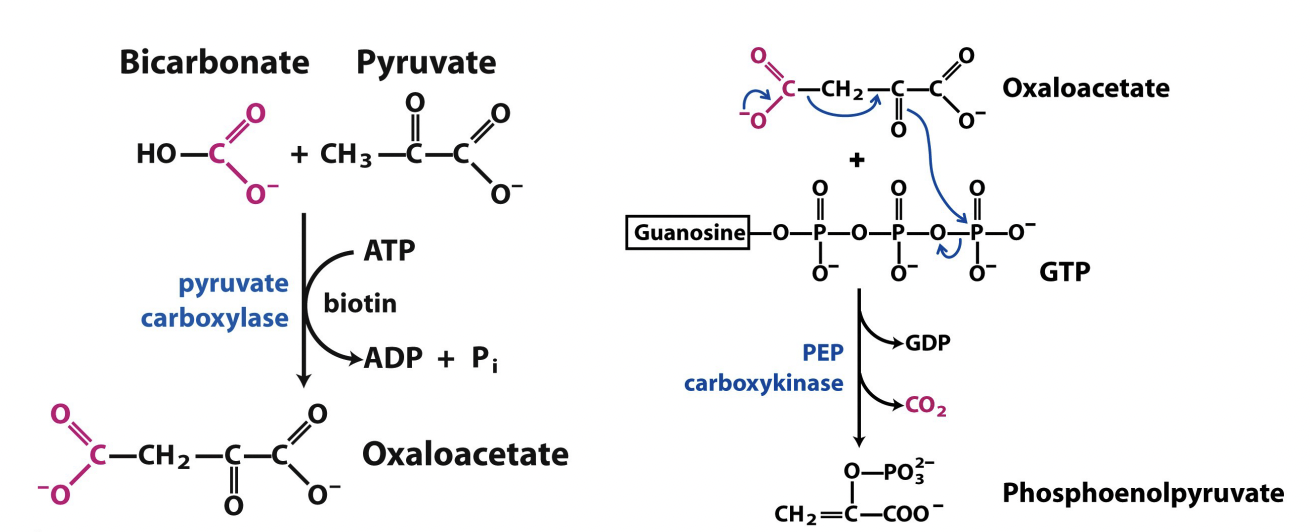
Synthesis of PEP from Pyruvate – reverse last step of glycolysis:
Bicarbonate + pyruvate
Pyruvate carboxylase takes CO2 off of bicarbonate, with ATP as a cofactor and adds it to the pyruvate to make oxaloacetate
Oxaloacetate - CO2
PEP carboxykinase removes the CO2 and adds a phosphate to generate phosphoenolpyruvate (PEP)
Fatty Acid Oxidation and Pyruvate Carboxylase:
Acetyl CoA levels high when beta oxidation is prominent
This inhibits PDH
Prevents wasteful oxidation of glucose
That same acetyl coa activates pyruvate carboxylase (first step in gluconeogenis)
This forms more oxaloacetate
This can be used in gluconeogenisis or put back into the krebs cycle
Need to replace oxaloacetate because if we kept using carbons from the kreb cycle for gluconeogenisis, eventually we would run out of carbons and oxaloacetate for the krebs cycle
Thats why carbon skeletons burned from amino acids
And why build up of acetyl-CoA doesn’t just turn off pyruvate dehydrogenase (PDH) but also switches on pyruvate carboxylase to turn pyruvate into oxaloacetate
Anaplerosis – replenishing an intermediate that have been extracted for biosynthesis
Oxaloacetate going back into the krebs cycle is called anaplerotic
Because we’ve been stealing intermediates from the krebs cycle to make more glucose