ORGANIC CHEM LAB
All organic compounds contain carbon atoms, hydrogen atoms, and one or more functional groups
Organic Chemistry: The study of carbon compounds
Covalent Bond: a chemical bond formed from the sharing of electrons
Name | Symbol | Number of Outer Electrons | Bonding Capacity |
Carbon | C | 4 | 4 |
Hydrogen | H | 1 | 1 |
Nitrogen | N | 5 | 3 |
Oxygen | O | 6 | 2 |
HOW TO FIND BONDING CAPACITY?
A straight line from an atom with nothing on the other end of it is one electron available
SKETCHES
Methyl Group:
CH3
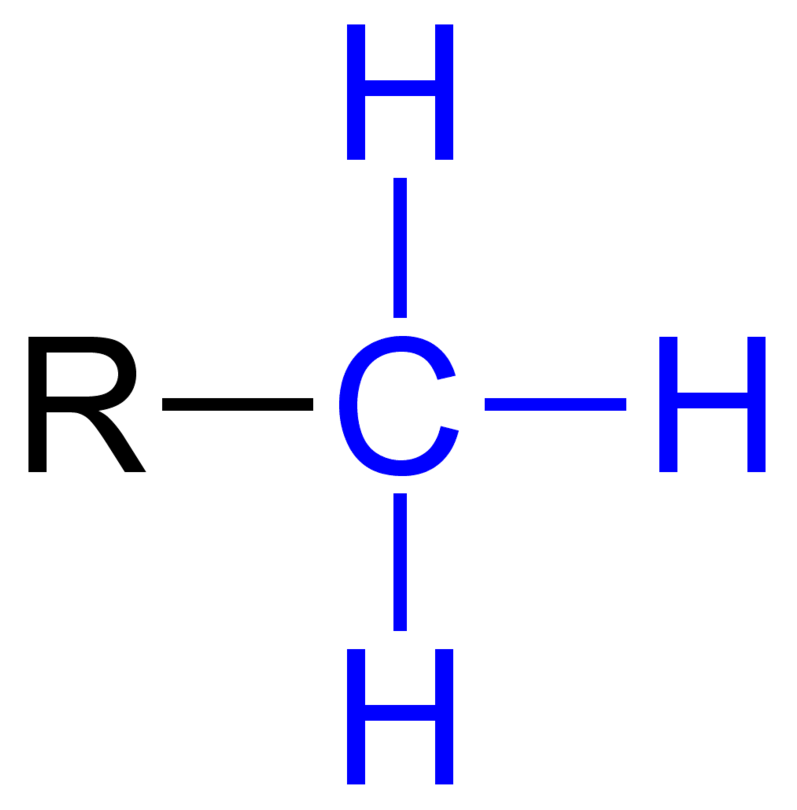
Amino:
NH2
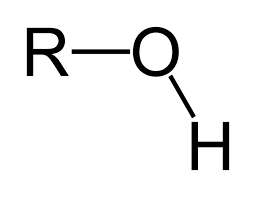
Hydroxl (Alcohol) Group:
OH
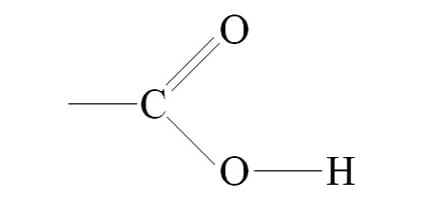
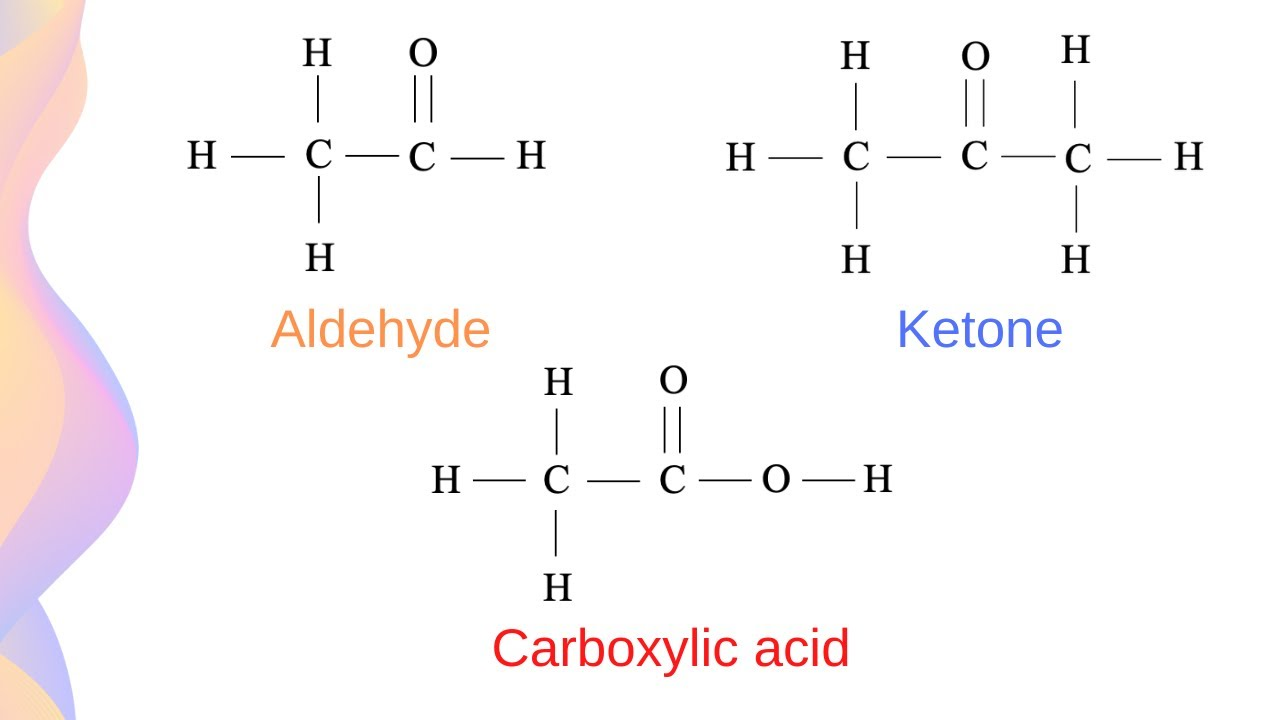
Carboxl Group or Carboxylic Acid:
COOH
Phosphate Group:
(H2)PO4
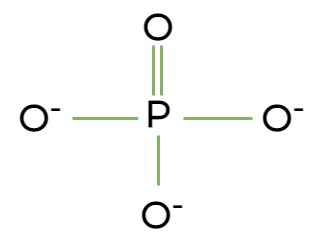
One of the Oxygens has a double bond*
Keytone + Aldehyde:
C=O
Both are C double bonded to an O
The difference is the location of the =O within the carbon chain:
Aldehyde: if the oxygen is on the end of a carbon skeleton
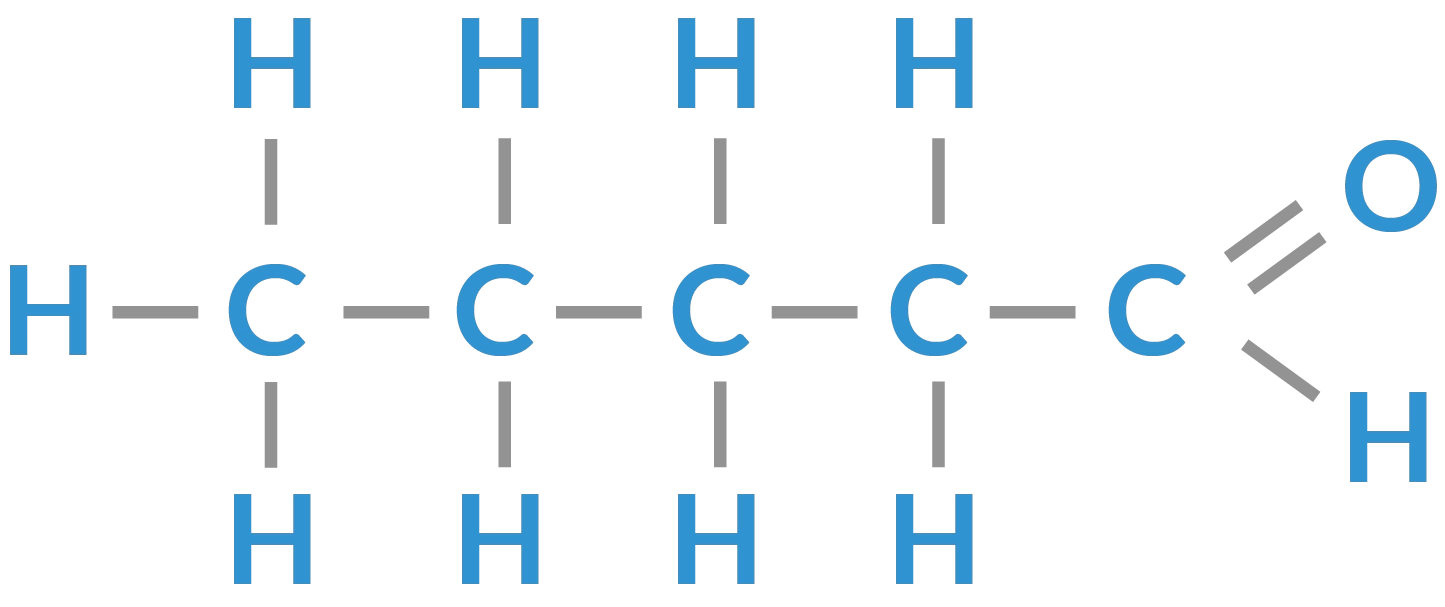
Ketone: if the oxygen is on a carbon in the middle of a skeleton
Carbohydrates:
General Formula of : CxH(2x)H(x)
Ratio of 1:2:1
Complex Carbs can be broken down into simple sugars such as monosaccharides
Simple sugars can be joined together to form disaccharide and polysaccharides
Simple Sugars: follow the 1:2:1 ratio rule
Complex usually one oxygen and 2 hydrogens short of the ration
Dehydration Synthesis:
When two simple sugars(which have the expected ration), react with each other and form a larger sugar molecule - they lose a water molecule from between them.
Sometimes labeled “condesation reaction”" as two small molecules are consolidated into one larger molecule
To find if mono, di, tri, saccharide:
Compare number of carbons and oxygens
Difference + 1 = number of sacchardies
Example: C9 H14 07
Two Water molecules missing, so trisacharide
Lipids:
Organic molecules that are insoluble
Amount of oxygen is much less that amount of carbon
Carb - “watered carbon” which implies near equal amount of C and O
Can be seperated into 3 categories: Fats, Phospholipids, steroids
Fats
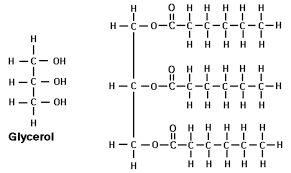
Lipid molecules composed of 2 kinds of building blocks:
A glycerol and 3 fatty acids
Glycerol:
3 carbon skeleton with 3 alcohol groups (OH) on each carbon
C3 H5 (OH)3
Fatty Acid:
Long carbon skeleton with a carboxylic acid (COOH) at one end
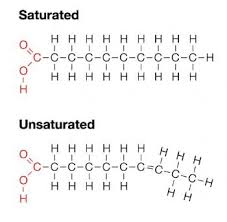
2 types: Saturated or Unsaturated
Saturated:
is completely “hydrogenated”
No double bonds
Unsaturated:
Double bond between carbon
Glycerol and fatty acids combine through dehydration synthesis
Phospholipids:
contains glycerol and fatty acids like fat, but one fatty acid chain is replaced by a:
complex group of atoms that contain a phosphate group
When fats or phospholipids are synthesized, they react with their compenent parts, and water is removed from between them
When being digested or broke down, opposite occurs
When water is added to a large molecule to break it down, the reaction is called Hydrolysis
Steriods:
complex lipids composed of 4 interlocking carbon rings
Proteins:
composed of amino acids
each amino acid is composed of a:
carbon skeleton
an amino group
a carboxylic acid
When 2 amino acids react with each other, water is removed
From hydrogen in the amino group
And OH from carboxylic acid
No oxygen left to hold them together
They now bond directly from the Nitrogen(N) and the Carbon(C) of the carboxylic acid.
This bond can only happen between amino acids and is called a Peptide Bond
A molecule consisting of several amino acids joined by peptide bonds are called polypeptides
Review Problems
MEMORIZE
C6 H10 O5 - Complex Carbohydrate
C3 H5 (OH)3 - Alcohol
C3 H7 (OH) - Alcohol
CH3 CO CH3 - Ketone
CH3 CH2 CHO - Aldehyde
CH3 CH2 - COOH - Carboxylic Acid
CH3 - (CH2)17 COOH - Carboxylic Acid
CH3 - CH - NH2 - Amino Acid
/. I
/ COOH
COOH CH2 NH2 - Amino Acid
Hydrolysis + Dehydration Synthesis:
Macromolecules - Large organic molecules
Formed by removal of water from 2 adjacent functional groups - Dehyradtion Synthesis
H - subunit - OH+H - subunit - OH ———→ H - subunit - subunit - OH + HOH
Macromolecules can be broken down by addition of water between 2 adjacent subunits - Hydrolysis
H - subunit - subunit - OH+H2O —————→ H - subunit - OH+H - subunit - OH
Amino Acid:
The base structure of an amino acid with an R group can be represented as follows:
H
|
H2N - C - COOH
|
R
Key Components:
1. Central Carbon (α-carbon): The central atom to which all other groups are attached.
2. Amino Group (-NH₂): A functional group that acts as a base, capable of accepting a proton.
3. Carboxyl Group (-COOH): A functional group that acts as an acid, capable of donating a proton.
4. Hydrogen Atom (H): A single hydrogen atom bonded to the central carbon.
5. Side Chain (R group): A variable group that determines the unique properties of the amino acid. Each of the 20 standard amino acids has a different R group, ranging from a simple hydrogen atom (as in glycine) to more complex structures like a benzene ring (as in phenylalanine).
This general structure makes amino acids versatile and essential for building proteins.