8.1 Nature of Acids and Bases
Arrhenius Theory of Acids and Bases
- acids dissociate to produce hydrogen ions (H+(aq))
- Ex: HCl, H2SO4
- bases dissociate to produce hydroxide ions (OH-(aq))
- Ex: NaOH, LiOH
- Drawbacks: only includes bases which contain hydroxide ions, and assumes all acid/base reactions occur in aqueous solutions.
- NH3 (g) + H2O → NH4 + OH
Bronsted-Lowry Theory
- An acid is a hydrogen ion donor
- Arrhenius theory predicts that dissolving hydrogen fluoride in water dissociates the molecule into a hydrogen ion and fluoride ion. However, the hydrogen ion is not stable, and it reacts with water to form hydronium (H3O(aq)).
- A base is a hydrogen ion acceptor.
- Instead of being hydroxide producers, they’re hydrogen acceptors.
- When ammonia dissolves in water, it pulls one of the hydrogen off the water making a hydroxide ion, giving the solution basic properties.
Conjugate Acid-Base Pairs:
Since the reaction can occur in the reverse direction, we can have an acid/base pair in the reverse direction (opposite as the forward direction).
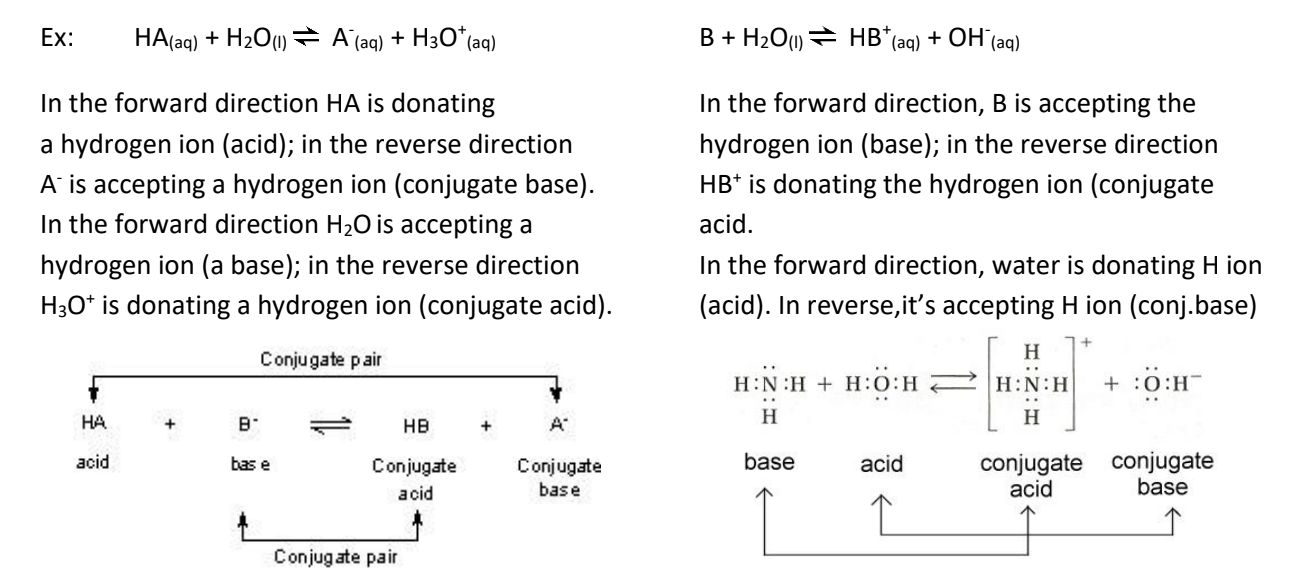
Amphiprotic (amphoteric) substances:
- Able to donate or accept a hydrogen ion, therefore it can act as an acid or a base.
- Ex: water.
The Acid Ionization Constant, Ka

We make two simplifications:
- Since water is a liquid, its concentration is a constant, therefore it becomes part of K.
- We can simplify the reaction by using hydrogen ions instead of hydronium ions:
