Chapter 4: Atomic Structure
4.1-Developing the Model of the Atom
Rutherford replaced the plum pudding model with the nuclear model
- In 1804, John Dalton agreed with Democritus that matter was made up of tiny spheres that couldn’t be broken up, but he reckoned that each element was made up of a different type of atom
- However, in 1909, scientists in Rutherford’s lab tried firing a beam of alpha particles at thin gold foil-this was the alpha scattering experiment.
- From the plum pudding model, they expected the particles to pass straight through the gold sheet, or only be slightly deflected.
- But although most of the particles did go straight through the sheet, some were deflected more than expected, and a few were deflected back the way they had come-something the plum pudding model couldn’t explain
- Nearly 100 years later, J.J.Thompson discovered particles called electrons that could be removed from atoms.
- So Dalton’s theory wasn’t quite right.
- Thomson suggested atoms were spheres of positive charge with tiny negative electrons stuck in them like fruit in a plum pudding
- the plum pudding model
- Because a few alpha particles were deflected back, the scientists realised that most of the mass of the atom must be concentrated at the centre in a tiny nucleus.
- This nucleus must also have a positive charge, since it repelled the positive alpha particles
- They also realised that because nearly all the alpha particles passed straight through, most of an atom is just empty space.
- This was the first nuclear model of the atom.
Which developed into the current model of the atom
- The nuclear model that resulted from the alpha particle scattering experiment was a positively charged nucleus surrounded by a cloud of negative electrons
- Niels Bohr said that electrons orbiting the nucleus do so at certain distances called energy levels.
- His theoretical calculations agreed with experimental data
- Evidence from further experiments changed the model to have a nucleus made up of a group of particles which all had the same positive charge that added up to the overall charge of the nucleus
- About 20 years after the idea of a nucleus was accepted, in 1932, James Chadwick proved the existence of the neutron, which explained the imbalance between the atomic and mass numbers
Current model of the atom
- The model is constantly being changed, but currently
- It contains protons and neutrons, which gives it an overall positive charge
- The rest of mostly empty space, negative electrons more around the outside of the nucleus really fast
- Radius of atom is 1x10(-10)
- Number of protons = number of electrons
- If they gain energy by absorbing EM radiation they move to a higher energy level
4.2-Isotopes and Nuclear Radiation
Isotopes are different forms of the same element
- All atoms of each element have a set number of protons.
- The number of protons in an atoms is its atomic number
- The mass number is the number of protons+neutrons
- Isotopes are atoms with same number of protons different number of neutrons
- All elements have different isotopes, but there are usually only one or two stable ones
- The other unstable isotopes tend to decay into other elements and give out radiation as they try to become more stable.
- This process is called radioactive decay
- Radioactive substances spit out one or more types of ionising radiation from their nucleus-the ones you need to know are alpha, beta and gamma radiation
- They can also release neutrons when they decay, as they rebalance their atomic and mass numbers
- Ionising radiation is radiation that knocks electrons off atoms, creating positive ions,
- The ionising power of a radiation source is how easily it can do this
Alpha particles are helium nuclei
- Alpha radiation is when an alpha particle is emitted from the nucleus.
- A a-particle is two neutrons and two protons
- They don’t penetrate very far into materials and are stopped quickly-they can only travel a few cm in air and are absorbed by a sheet of paper
- Because of their size they are strongly ionising
Beta particles are high-speed electrons
- A beta particles, is simply a fast-moving electron released by the nucleus. Beta particles have virtually no mass and a charge of -1
- They are moderately ionising.
- They penetrate moderately far into materials before colliding and have a range in air of a few meters.
- They are absorbed by a sheet of aluminium
- For every beta particle emitted, a neutron in the nucleus has turned into a proton
Gamma rays are EM waves with a short wavelength
- Gamma rays are waves of electromagnetic radiation released by the nucleus
- They penetrate far into materials without being stopped and will travel a long distance through air
- This means they are weakly ionising because they tend to pass through rather than collide with atoms.
- Eventually they hit something and do damage
- They can be absorbed by thick sheets of lead or metres of concrete
4.3-Nuclear Equations
Mass and atomic numbers have to balance
- Nuclear equations are a way of showing radioactive decay by using element symbols
- They’re written in the form:atom before decay - atom after decay+radiation emitted
- There is one golden rule to remember:
- the total mass and atomic number must be equal on both sides
Alpha decay decreases the charge and mass of the nucleus
- Remember, alpha particles are made up of two protons and two neutrons.
- So when an atom emits an alpha particles, its atomic number reduces by 2 and its mass number reduces by 4
- A proton is positively charges and a neutron is neutral, so the charge of the nucleus decreases
- In nuclear equations, an alpha particles can be written as a helium nucleus
Beta decay increases the charge of the of the nucleus
- When beta decay occurs, a neutron in the nucleus turns into a proton and releases a fast-moving electron
- The number of protons in the nucleus has increased by 1.
- This increases the positive charge of the nucleus
- Because the nucleus has lost a neutron and gained a proton during beta decay, the mass of the nucleus doesn’t charge
- A beta particle is written as 0/-1e in nuclear equations
Gamma rays don’t change the charge or mass of the nucleus
- Gammas rays are a way of getting rid of excess energy from a nucleus
- This means that there is no change to the atomic mass or atomic number of the atom
4.4-Half-life
Radioactivity is a totally random process
- Radioactive substances give out radiation from the nuclei of their atoms-no matter what
- This radiation can be measured with a Geiger-Muller tube and counter, which records the count-rate-the number of radiation counts reaching it per second
- Radioactive decay is entirely random. So you can’t predict exactly which nucleus in a sample will decay next, or when any one of them will decay
- But you can find out the time it takes for the amount of radiation emitted by a source to halve, this is known as the half-life.
- It can be used to make predictions about radioactive sources, even though their decays are random
- Half-life can be used to find the rate at which a source decays-its ACTIVITY. Activity is measured in becquerels, Bq
The radioactivity of a source decreases over time
- Each time a radioactive nucleus decays to become a stable nucleus, the activity as a whole will decrease
- For some isotopes it takes just a few hours before nearly all the unstable nuclei have decayed, whilst others last for millions of years
- The problem with trying to measure this is that the activity never reaches zero, which is why we have to use the idea of half-life to measure how quickly the activity drops off
- The half-life is the time taken for the number of radioactive nuclei in an isotope to halve
- It is also the time taken for the activity, and so count-rate, to halve.
- A short half-life means the activity falls quickly, because the nuclei are very unstable and rapidly decay.
- Sources with a short half-life are dangerous because of the high amount of radiation they emit at the start, but they quickly become safe
- A long half-life means the activity falls more slowly because most of the nuclei don’t decay for a long time-the source just sits there, releasing small amounts of radiation for a long time.
- This can be dangerous because nearby areas are exposed to radiation for millions of years
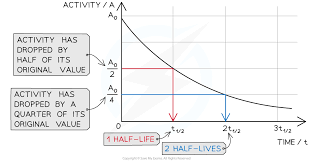
You can measure half-life using a graph
If you plot a graph of activity against time, it will always be shaped like the one to the right
The half-life is found from the graph by finding the time interval on the bottom axis corresponding to a halving of the activity on the vertical axis
4.5-Background Radiation and Contamination
Background Radiation comes from many sources
Background radiation is the low-level radiation that’s around us all the time. You should always measure and subtract the background radiation from your results. It comes from:
- Radioactivity of naturally occuring unstable isotopes which are all around us-in the air, in food, in building materials and in the rocks under our feet
- Radiation from space, which is known as cosmic rays.
- These come mostly from the sun.
- Luckily, the earth’s atmosphere protects us from much of this radiation
- Radiation due to human activity or nuclear waste exists.
- But this represents a tiny proportion of the total background radiation
- The radiation doss tells you the risk of harm to body tissues due to exposure to radiation. It’s measured in sieverts.
- The dose from background radiation is small, so millisieverts are often used.
- Your radiation dose varies depending on where you live or if you have a job that involves radiation
Exposure to radiation is called irradiation
- Objects near a radioactive source are irradiated by it.
- This simply means they’re exposed to it
- Irradiating something does not make it radioactive
- Keeping sources in lead-in boxes, standing behind barriers or being in a different room and using remote-controlled arms are all ways of reducing the effects of irradiation
Contamination is radioactive particles getting onto objects
- If unwanted radioactive atoms get onto or into an object, the object is said to be contaminated.
- These contaminating atoms might then decay, releasing radiation which could cause you harm
- Contamination is especially dangerous because radioactive particles could get inside your body
- Gloves and tongs should be used when handling sources, to avoid particles getting stuck to your skin or under your nails.
- Some industrial workers wear protective suits to stop them breathing in particles
The seriousness of irradiation and contamination depends on the source
Contamination or irradiation can cause different amounts of harm
- Outside the body, beta and gamma sources are the most dangerous.
- This is because beta and gamma can penetrate the body and get to the delicate organs.
- Alpha is less dangerous because it can’t penetrate the skin and is easily blocked by a small air gap.
- High levels of irradiation from all sources are dangerous, but especially from ones that emit beta and gamma
- Inside the body, alpha sources are the most dangerous, because they do all their damage in a very localised area.
- So contamination, rather than irradiation, is the major concern when working with alpha sources.
- Beta sources are less damaging inside the body, as radiation is absorbed over a wider area, and some passes out of the body although.
- Gamma sources are the least dangerous inside the body, as they mostly pass straight out-they have the lowest ionising power
- The more we understand about how radiation affects our bodies, the better we can protect ourselves when using it.
4.6-Uses and Risk
There are risks to using radiation
- Radiation can enter living cells and ionise atoms and molecules within them, this can lead to tissue damage
- Lower doses tend to cause minor damage without killing the cells.
- This can give rise to mutant cells which divide uncontrollably.
- This is cancer.
- Higher doses tend to kill cells completely, causing radiation sickness if a lot of cells all get blatted at once
Gamma sources are usually used in medical tracers
- Certain radioactive isotopes can be injected into people and their progress around the body can be followed using an external detector.
- A computer converts the reading to a display showing where the strongest reading is coming from
- One example is the use of iodine-123, which is absorbed by the thyroid gland just like normal iodine-127, but it gives out radiation which can be detected to indicate whether the thyroid gland is taking in iodine as it should
- Isotopes which are taken into the body like this are usually GAMMA, so that the radiation passes out of the body without causing much ionisation.
- They should have a short half-life so the radioactivity inside the patient quickly disappears
Radiotherapy-treating cancer with radiation
- Since high doses of ionising radiation will kill all living cells, it can be used to treat cancers
- Gamma rays are directed carefully and at just the right dosage to kill the cancer cells without damaging too many normal cells.
- Radiation-emitting implants can also be put next to or inside tumours
- However, a fair bit of damage is inevitably done to normal cells, which makes the patient feel very ill.
- But if the cancer is successfully killed off in the end, then it’s worth it
You have to weigh up the risks and benefits
- Risks: prolonged exposure to radiation poses future risks and causes side effects
- Benefits: can get rid of cancer entirely
- Perceived risk can vary from person to person
4.7-Fission and Fusion
Nuclear fission-splitting a large, unstable nucleus
- Nuclear fission is a type of nuclear reaction used to release energy from large and unstable atoms by splitting them into smaller atoms
- Spontaneous fission rarely happens.
- Usually, the nucleus has to absorb a neutron before it will split
- When the atom splits it forms two new lighter elements that are roughly the same size
- Two or three neutrons are also released when an atom splits.
- If any of these neutrons are moving slow enough to be absorbed by another nucleus, they cause more fission to occur, which is a chain reaction
- The energy not transferred to the kinetic energy is carried away by gamma rays
- Energy carried away can be used to heat water making steam to turn turbines and generators
- The amount of energy produced by fission in a nuclear reactor is controlled by changing how quickly the chain reaction can occur, which is done by control rods
- Uncontrolled chain reactions quickly lead to lots of energy being released as an explosion-this is how nuclear weapons work
Nuclear fusion-joining small nuclei
- Nuclear fusion is the opposite of nuclear fission
- Two light nuclei collide at high speed and join to create larger, heavier nucleus
- Heavier nucleus produced does not have as much mass as the two separate light nuclei did.
- Some of the mass is converted to energy, which is released as radiation
- Fusion released a lot of energy
- Scientists haven’t found a way of using fusion to generate energy, and would be to hard and expensive to do at the moment