Unit 5 IB HL Biology
ATP
Vocabulary:
ATP hydrolysis -
Phosphorylation -
Autotrophs -
Photoautotrophs -
Trophic level -
The name for Adenosine Triphosphate
ATP is a modified nucleotide
Nucleotides are the monomers for DNA and RNA
ATP is produced by the mitochondria during aerobic respiration
ATP is required in cells to transfer energy wherever it is needed
ATP is often referred to as the universal energy currency of cells
Cellular energy
ATP energy is used for cellular processes that require energy, such as:
Active transport
Anabolic reactions
Muscle contractions
Movement of cells or parts within cells
etc…
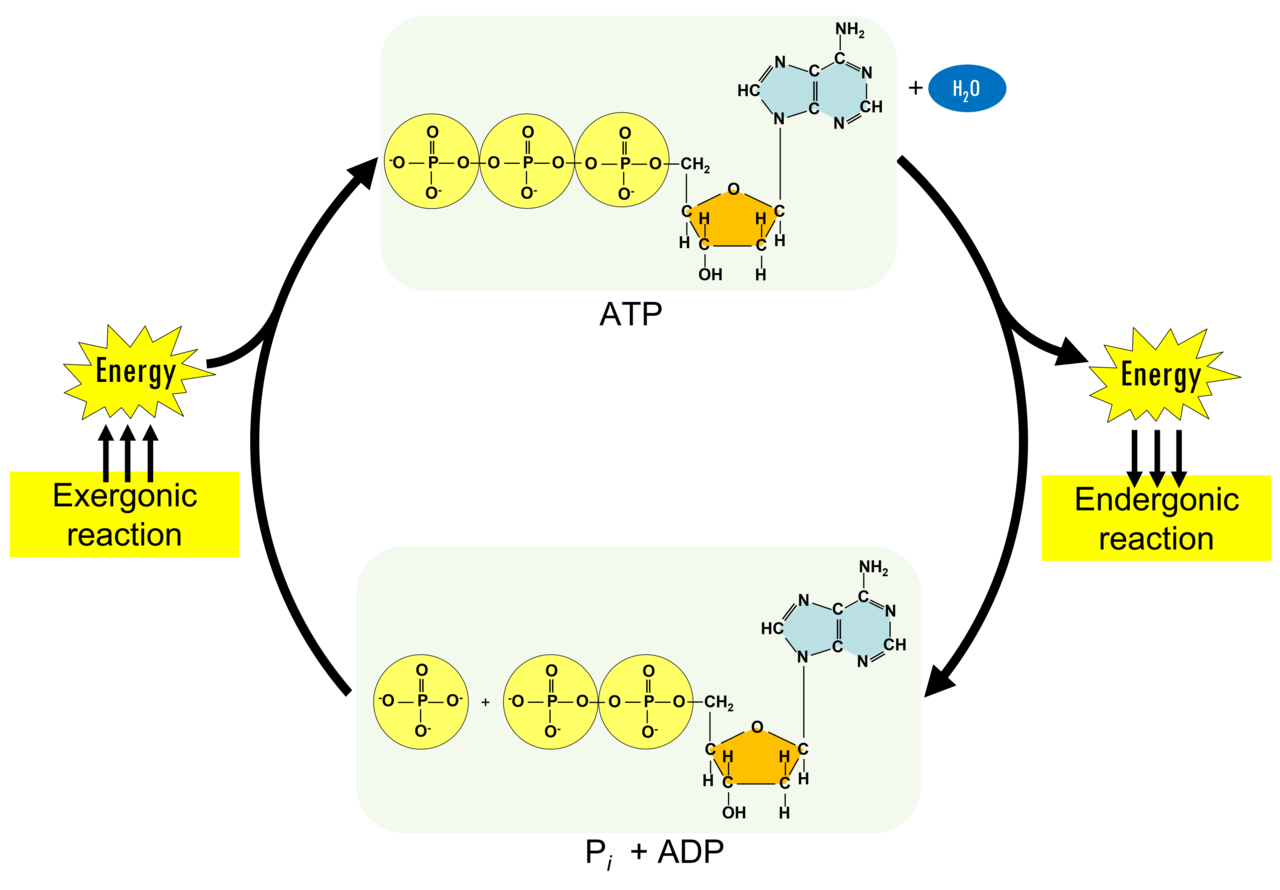
Structure of ATP
ATP has three components:
Adenine (nitrogenous base - orange rectangle)
Ribose (pentose sugar - blue pentagon)
3 Phosphate groups (Functional group - purple circles)
Energy
The three phosphate groups all have a negative charge
The negative charges repel each other
The phosphate groups don’t want to be next to each other and are constantly trying to break away
ATP storing energy
By creating bonds between the three phosphate groups it is compressing them together like a spring.
Releasing energy
The energy is released when the third bond is broken
The process of breaking the bond between the 2nd and 3rd phosphate is called ATP hydrolysis
Hydro - water
Lysis - breaking
ATP hydrolysis is an exergonic reaction
Exergonic reaction - A spontaneous reaction wherein energy is released into the surrounding environment
ATP hydrolysis creates:
ADP (Adenosine diphosphate)
Pi (Phosphate)
ATP hydrolysis
This is an exergonic reaction because the energy is released
A water molecule is used to break the bond between the phosphate groups
The phosphate group that is released often attaches to another molecule
Adenosine diphosphate
ADP contains some energy but not as much as ATP. Since ADP only contains two phosphate groups the repel force is not as strong leading to less energy being created.
ADP phosphorylation
This is an endergonic reaction because the energy is temporarily stored
Phosphorylation is an endergonic reaction
Endergonic reaction - Non-spontaneous reactions wherein energy is absorbed from the surrounding environment
ADP can be turned into ATP by adding a phosphate group and removing a water molecule. This is known as phosphorylation
ATP - ADP cycle
Photosynthesis
Vocabulary:
Photolysis - The splitting of water molecules using light energy during the light-dependent reactions of photosynthesis, releasing oxygen, electrons, and protons
Photons - The fundamental unit or particle of light. Photons have no mass and travel at the speed of light
Light-dependent reactions - The initial stages of photosynthesis that occur in the thylakoid membranes of chloroplasts. These reactions utilize light energy to convert ADP and Pi into ATP, and reduced NADP to reduced NADP. They also generate oxygen through the process of photolysis.
Light-independent reactions - The second stage of photosynthesis. These reactions occur in the stroma of chloroplasts and involve the fixation of carbon dioxide and the production of carbohydrates using the ATP and reduced NADP generated in the light-dependent reactions
Autotrophs -
Phototrophs -
Trophic level -
Thylakoids - Flattened, membrane-bound sacs in chloroplasts that contain the photosynthetic pigments and proteins necessary for the light-dependent reactions of photosynthesis
Chloroplasts - Organelles found in some plant cells. They contain chlorophyll and carry out the process of photosynthesis
Stroma - The space between the inner membrane and thylakoid membranes in chloroplasts containing enzymes and products for the Calvin cycle
Action spectrum - A graphical representation that shows the effectiveness of different wavelengths of light in driving a specific physiological or biochemical process, such as photosynthesis
Chlorophyll - A green pigment found in the chloroplasts of plants and algae that plays a central role in photosynthesis by absorbing light energy.
Accessory Pigments - Additional pigments found in chloroplasts that assist chlorophyll in capturing light energy during photosynthesis. These pigments broaden the absorption spectrum, allowing plants to capture light energy from a wider range of wavelengths
Absorption spectrum - The wavelengths of light absorbed by a particular substance or pigment
Light reaction -
Calvin cycle - A series of biochemical reactions that occur in the stroma of chloroplasts during the light-independent reactions of photosynthesis. The Calvin cycle involves the fixation of carbon dioxide, reduction of carbon compounds, and regulation of RuBP, leading to the production of carbohydrate
Photosystems - Large protein complexes found in the thylakoid membranes of chloroplasts. They are involved in the light-dependent reactions of photosynthesis and contain pigments, including chlorophyll and accessory pigments, that capture light energy and initiate the electron transport chain
Reaction Center - A specific protein complex within a photosystem where light energy is converted into chemical energy. It contains specialized chlorophyll molecules that can donate electrons directly to the electron transport chain
Photoactivation - The activation of a molecule or system through the absorption of light energy. In the context of photosynthesis, it typically refers to the activation of chlorophyll and other pigment, which trigger subsequent biochemical reactions.
Photophosphorylation - The process of generating ATP using light energy. It occurs during the light-dependent reactions of photosynthesis, where light energy is used to phosphorylate ADP to ATP
Electron Transport Center - A series of protein complexes in the inner membrane of the mitochondria that transfer electrons and pump protons to create a proton gradient
Concentration gradient -
Chemiosmosis - The process by which energy stored in the proton gradient is used to produce ATP.
ATP synthase -
Cyclic phosphorylation -
Non-cyclic phosphorylation -
Carbon fixation - The conversion of inorganic carbon (carbon dioxide from the atmosphere) to organic carbon by a living organism
Rubisco - An enzyme that catalyzes the addition of carbon dioxide to RuBP during the Calvin Cycle. It is the most abundant enzyme on Earth and plays a critical role in carbon fixation
Photorespiration -
Energy cannot be created or destroyed, it can only be transferred and transformed
Photosynthesis transforms light energy (from the sun) and turns it into chemical energy (glucose and other organic molecules).
Photosynthetic organisms
Organisms have developed the ability to absorb this light energy from the sun and convert it into chemical energy in the form of glucose and other carbon compounds, thus providing the energy for almost all ecosystem on the planet
Autotrophs- organisms that can produce their own chemical energy (organic compounds).
Autotrophs are also known as producers.
Supply chemical energy to the entire ecosystem
Every organism, regardless of its trophic level, relies on the energy from the sun that is converted using photosynthesis.
Primary consumers eat the producers and absorb the energy they have stored
Secondary consumers then eat the primary consumers and the food chain continues
Examples:
Plants
Algae
Cyanobacteria
Photoautotrophs- use light to produce their chemical energy (they perform photosynthesis).
Producers absorb light and produce glucose and other carbon compounds
Photosynthesis Equation
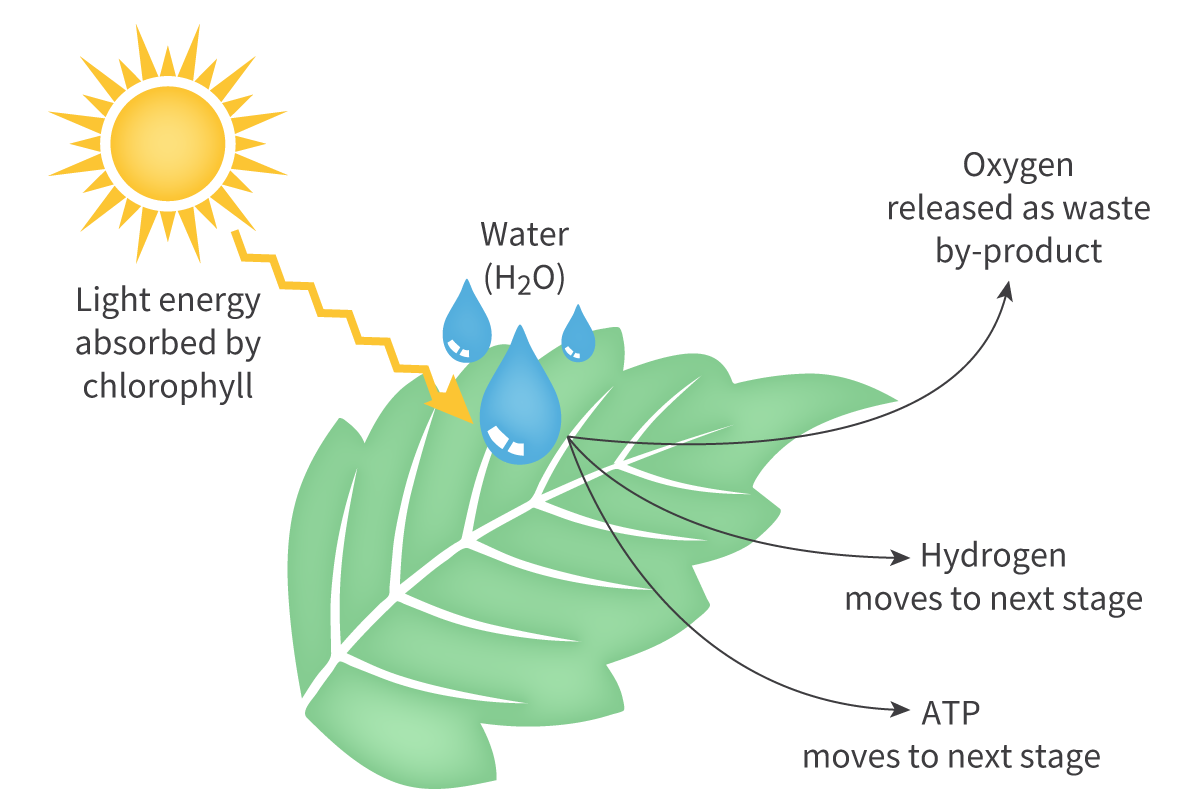
Water is used in photosynthesis because a source of hydrogen is required to convert CO into glucose
Water is the most accessible source of hydrogen on Earth
To access the hydrogen it must go through photolysis
The energy in photons is used to split water molecules, generate hydrogen ions, electrons, and oxygen
Photolysis is the process of breaking up molecules using light
2H2O + photons → 4H+ + O2 + 4e–
Photo = light
lysis = break
Chloroplast structure
Has a double membrane surrounding the organelle
Outer membrane
Inner membrane
Thylakoids
Flattened, membrane-bound sacs
Surrounded by the thylakoid membrane
Thylakoid space - the inner region of the thylakoid
Because of its size and shape, it has a high SA: V ration
Contains chlorophyll
Location of light reaction
Arranged in stacks called grana to maximize light absorption
Stroma
Fluid-filled space between the inner membrane
Containing enzymes and materials for the Calvin Cycle
Thylakoid = stack of pancakes
Stroma = Syrup
Photosynthetic pigments
Pigments are molecules that absorb light
Wavelengths that are not absorbed are reflected - this is the color that we perceive
Major photosynthetic pigment = chlorophyll
Accessory pigments include xanthophyll and carotenoids
Chlorophyll
Chlorophyll is the main pigment found in plants
Chlorophyll a and chlorophyll b reflect green light and absorb most of the other colors of light (wavelengths)
Causes chloroplast to appear green
Absorption spectrum
the wavelengths of light absorbed by a particular substance or pigment
Visualized in a graph
Peaks in the graph represent high absorption
Each absorption spectrum line represents one type of pigment
Plants have several different types of pigments, so the overall rate of photosynthesis is a result of a combination of all of the pigments absorbing light
More light absorption by pigments increases the rate of photosynthesis
Paper chromatography
A lab technique to separate pigments as they move up the paper
Pigments will dissolve in the solvent and will separate based on their solubility
2 Halves of photosynthesis
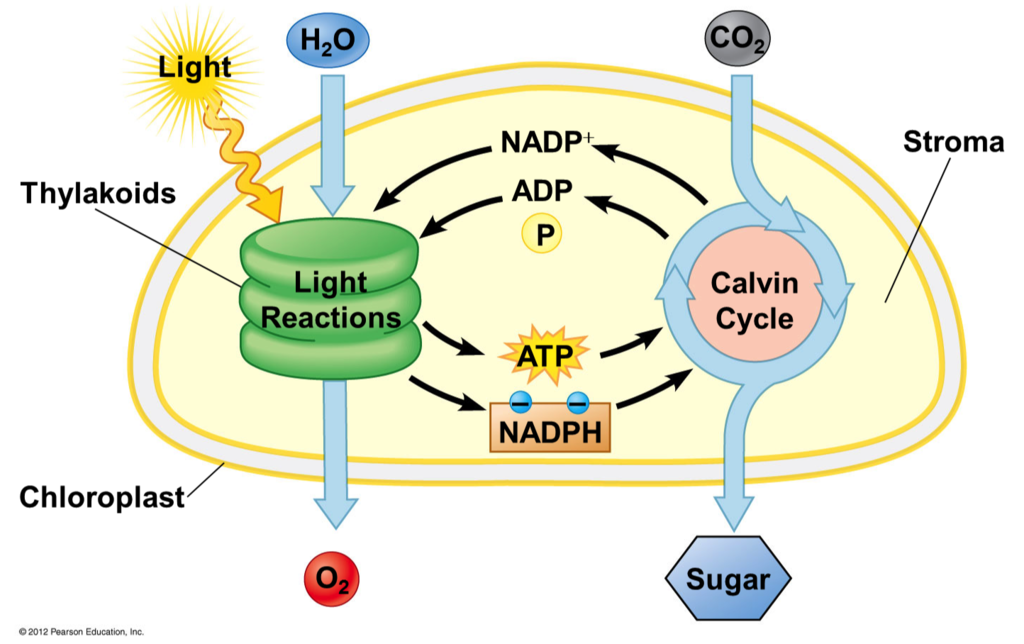
Light-dependent reactions
Light reaction
Occurs in the thylakoids
Utilizes photosynthetic pigments to absorb light
Light energy splits H2O and produces O2 as a byproduct
Creates ATP and NADPH to be used by the Calvin Cycle
Light-independent Reactions
Calvin Cycle
Occurs in the stroma
Uses ATP and NADPH from the light reaction
Carbon fixation of CO2 from the atmosphere
Produces Sugars
Reduction and Oxidation
Reduction
Gaining electrons
The charge is “reduced” because it becomes more negative
Oxidation
Losing electrons
Light Reaction
Photosystems
Integral protein complexes located within the phospholipid bilayer
In chloroplast: the thylakoid membrane
In cyanobacteria: the cell membrane
Photosystems contain chlorophyll that will absorb light energy
Photosynthesis occurs first within the photosystems.
Photosystems - Large protein complexes found in the thylakoid membranes of chloroplasts. They are involved in the light-dependent reactions of photosynthesis and contain pigments, including chlorophyll and accessory pigments, that capture light energy and initiate the electron transport chain.
Reaction Center - A specific protein complex within a photosystem where light energy is converted into chemical energy. It contains specialized chlorophyll molecules that can donate electrons directly to the electron transport chain.
There are two types of photosystems:
Photosystem 2
Sensitive to 680nm of light
Photosystem 1
Sensitive to 700nm of light
The light reaction is all about the flow of electrons.
Photoactivation in photosystems
Photoactivation - The activation of a molecule or system through the absorption of light energy. In the context of photosynthesis, it typically refers to the activation of chlorophyll and other pigments, which triggers subsequent biochemical reactions.
Photons of light strike the pigment molecules within the photosystem
Excite the electrons within these molecules
Excited electrons are transferred between the array of pigments within the photosystem
Excited electrons finally reach the reaction center - a special chlorophyll A molecule
At the reaction center, the excited electron will be emitted from the photosystem
The photosystem has become oxidized (lost an electron)
Photosystem 2
Photosystem 2 is the first photosystem to undergo photoactivation
After the electron is emitted from photosystem 2, it is transferred from the reaction center to the first electron transport chain (ETC)
Now PS2 is missing an electron (it is oxidized) - this is very unstable
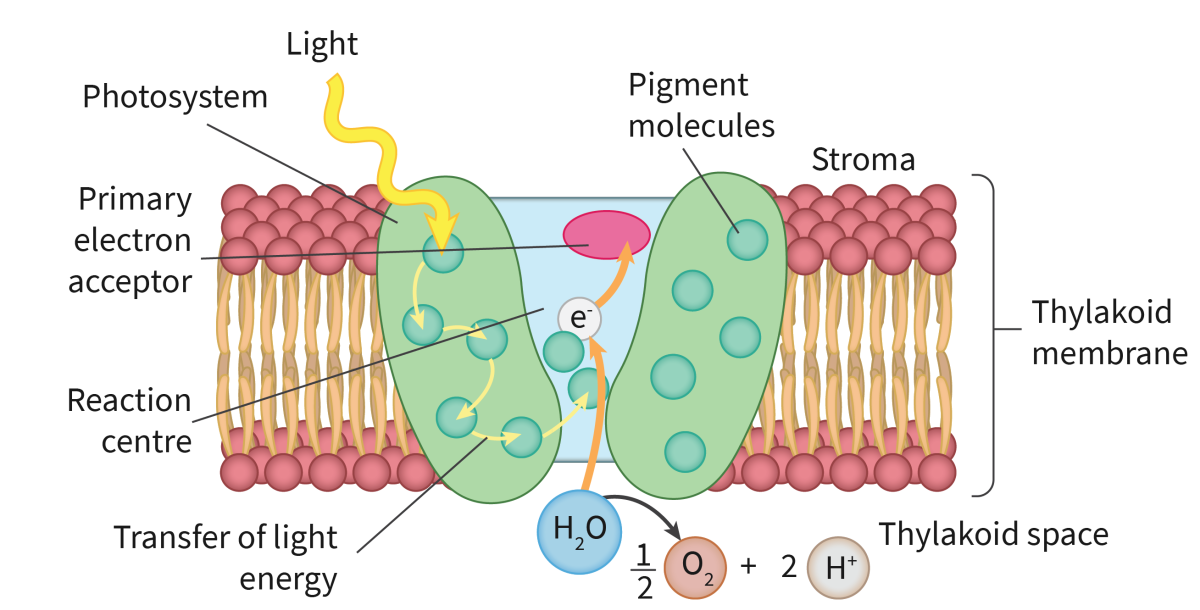
Photolysis in Photosystem 2
Electrons are replaced during the process of photolysis
Photolysis is the process of using light energy to break water molecules to replace missing electrons in PS2
Equation: 2H2O → 4H+ + O2 + 4e-
Photolysis occurs in the thylakoid space by PS2
H+ (protons) remain in the thylakoid space, beginning to build a concentration gradient
O2 diffuses out of the chloroplast →cell →leaf
e- (electrons) are transferred to PS2
Electron transport chain (ETC)
A series of integral protein complexes within the thylakoid membrane
The first ETC receives excited electrons from PS2
There are 2 functions of the first ETC:
Transfer electrons from PS2 to PS1
Harness the extra energy from excited electrons & use it to pump H+ (protons) into the thylakoid space - This establishes a proton concentration gradient: high (H+) in thylakoid
Proton concentration gradient
High concentration of the H+ in the thylakoid space for 3 reasons:
H+ produced in the thylakoid during photolysis
H+ pumped into the thylakoid by the first ETC
Thylakoids are small spaces so H+ accumulates quickly
Chemiosmosis
The proton concentration gradient allows for passive transport of protons OUT of the thylakoid (down its concentration gradient)
Can the protons (H+) pass through the membrane unassisted via simple diffusion? NO!
Because of their charge, protons (H+) can only exit the thylakoid via transmembrane integral protein
Chemiosmosis is the diffusion of H+ down its concentration gradient
ATP synthase
Transmembrane integral protein that is also an enzyme (-ase)
ATP synthase performs phosphorylation to create (synthesize) ATP
This process requires energy - where is the energy coming from?
Chemiosmosis drives ATP synthesis
As the H+ diffuses through ATP synthase, it causes the enzyme to turn - much like a water wheel creating power
This provides the energy needed to phosphorylate ADP into ATP
This process is ultimately driven by light: Phosphorylation
ATP made during phosphorylation will go to power the Calvin Cycle
Photosystem 1
Photoactivation in PS1
Photoactivation occurs in PS1
Excited electrons are:
Transferred between the pigments & and end up in the reaction center
Emitted from the reaction center and are transferred to an enzyme called NADP+ reductase
Replacing electrons in PS1
After the excited electrons are emitted from PS1, they need to be replaced
Electrons traveling from PS2 via the 1st ETC will replace the missing electrons from PS1
NADP+/NADPH
NADP+ (Nicotinamide adenine dinucleotide phosphate) is an electron carrier
NADP+ is the oxidized form (“empty” of electrons)
When NADP+ picks up electrons it becomes reduced (NADPH, “full” of electrons)
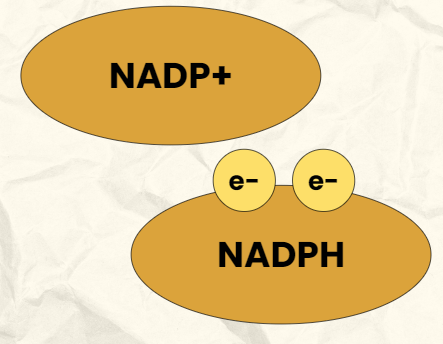
Reduction of NADP+
Electrons leave PS1 and are transferred to NADP+ reductase
NADP+ reductase is an enzyme that combines the electrons with NADP+ to NADPH
This process reduces NADP+ into NADPH
Occurs on the stroma side of the thylakoid membrane
The NADPH produced (“filled”) during the light reaction will go to the Calvin Cycel to drop off the electrons
Non-cyclic Photophosphorylation
The process of phosphorylation that was described earlier is non-cyclic photophosphorylation
Electrons flow from:
Water → PSII → 1st ETC → PSI → NADPH
ATP is generated as a result of the 1st ETC’s function
Cyclic photophosphorylation
The thylakoid membranes contain thousands of PS2s, PS1s, ETC’s, and ATP synthases
Sometimes electrons that are emitted from PS1 are transferred back to the 1st ETC (instead of NADP+ reductase)
The electrons travel from:
PSI → 1st ETC → PSI
When this happens ATP is made like normal because the ETC still creates the proton gradient
What makes this “cyclic” is the pathway that the electrons take: electrons are lost from and return to the same photosystem
Light Reaction Summary
Pigments harness light energy to excite electrons that will eventually reduce NADP+ into NADPH
ATP is synthesized using photophosphorylation
O2 is produced as a byproduct of the photolysis of H2O
ATP and NADPH will go into the Calvin Cycle
The light reaction is also called the light-dependent reaction
Calvin Cycle
The Calvin Cycle is also called the light-independent reaction
Occurs in the stroma
CO2 is “fixed” from the atmosphere and converted into sugars (organic molecules)
Utilizes the energy from ATP and electrons from NADPH (both of which were made in the light reaction)
Cyclical metabolic pathway
There are three phases
Carbon fixation
Reduction (synthesis of triose phosphate)
Regeneration
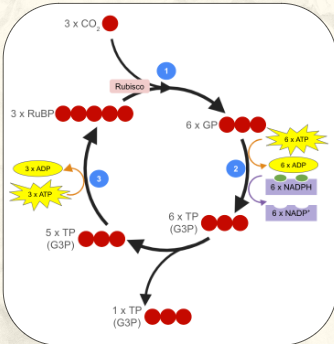
Carbon fixation
The process of attaching a CO2 to a 5-carbon sugar called ribulose (RuBP)
3 CO2 + 3 RuBP total per “turn”
Carbon fixation is catalyzed by an enzyme called Rubisco
Rubisco is the most abundant enzyme on Earth
After CO2 has been “fixed” to RuBP, each of the resulting 6 carbon compounds breaks into 2 × 3 carbon compounds called glycerate 3 phosphate (GP)
This creates a total of 6 x GP
Rubisco
Rubisco is found in really high concentrations in the stroma because:
Rubisco is a “slow” enzyme and doesn’t work very efficiently
There is a very high energy requirement for the Calvin Cycle
Rubisco can mistakenly add an O2 instead of a CO2
That makes Rubisco even less efficient
If an O2 is added, the molecule can no longer proceed through the rest of the Calvin Cycle (this is called photorespiration)
Rubisco works best in high concentrations of CO2 to reduce the chance of an O2 being mistakenly added to RuBP
Reduction
Each GP molecule is converted into a triose phosphate (TP) called Glyceraldehyde 3 phosphate (G3P)
Each molecule requires:
The energy from 1 ATP molecule
The electrons from 1 NADPH molecule
This is a total of:
6 x GP converted into 6 TP (G3P)
6 x ATP used (creates 6 x ADP)
6 x NADPH used (creates 6 x NADP+
It is called reduction because GP gains electrons as it is converted into TP
Leaving the cycle
At the end of the reduction phase, there are 6 x TP (G3P)
One triose phosphate (G3P) will exit the cycle
Five triose phosphate (G3P) will remain in the cycle and go into the 3rd phase: regeneration
Regeneration
Five triose phosphates (G3P) remain in the cycle (this is a total of 15 carbons)
The carbons in the 5 x TP will get rearranged into 3 x RuBP
This requires the energy from 3 x ATP (creates 3 x ADP)
The regeneration phase of the Calvin cycle recreates the CO2 receptor (RuBP). This allows the cycle to continue in a cyclical manner.
Making Glucose
The triose phosphate (G3P) will get turned into glucose after it exits the Calvin Cycle.
Making other Organic molecules
The triose phosphates that exit the Calvin Cycle can also be used to create any other organic molecule that plants need to synthesize.
Examples:
Other carbohydrates
Amino acids
Nucleotides
Limiting Factors of Photosynthesis
The rate of photosynthesis is limited by:
Light intensity (brightness)
CO2 concentration
Temperature
The wavelength of light also impacts photosynthesis
Cellular respiration
Vocabulary:
Exergonic -
Catabolic -
Heterotrophs -
Aerobic respiration -
Anaerobic respiration -
Glycolysis -
Link reaction -
Kreb cycle -
Oxidative phosphorylation -
Producing ATP from organic molecules
All living things must have some way to extract energy out of organic molecules to produce ATP
Photosynthetic organisms (autotrophs) make the organic molecules first, then they break them down
Heterotrophs - organisms that must get their energy from other organisms because they cannot produce their own organic molecules
Can be prokaryotic or eukaryotic
Example:
Humans
E.coil
Fungi
Breaking down organic molecules is catabolic and exergonic
The controlled release of energy stored in organic molecules is harnessed to produce ATP
Aerobic vs Anaerobic respiration
Aerobic respiration: utilizing O2 while breaking down organic molecules
Anaerobic respiration: Not utilizing O2 while breaking down organic molecules
Cellular respiration is used to refer to aerobic respiration
Mitochondrial Structure
Double membrane
Inner membrane
Highly folded
Cristae are the folds of the inner membrane
Outer membrane
Intermembrane space
Space between the two membranes
Very small - allows for easy accumulation (high concentrations) of protons
Mitochondrial matrix
Space inside of the inner membrane
Separate space with ideal pH and enzymes for specific reactions
Cellular respiration equation
Redox reactions
Cellular respiration is a series of redox reactions
4 Stages of cellular respiration
Glycolysis
Link reaction
Kreb cycle
Oxidative phosphorylation (OxPhos)
Stage 1 = aerobic
Stage 2-4 = anaerobic
Glycolysis
The process of breaking glucose into 2 pyruvate molecules
Occurs in the cytoplasm of cells
Prokaryotes and eukaryotes can perform glycolysis
Thought to be one of the most ancient cellular pathways
Glycolysis will occur in the presence and absence of oxygen (O2) - anaerobic
Glycolysis is a linear metabolic pathway
Series of 10 reactions each catalyzed by their own enzyme
Remember: we need a controlled release of energy = lots of small steps/reaction
There are 4 events of glycolysis
Phosphorylation
Lysis
Oxidation
ATP formation
Glycolysis: Energy investment phase
2 ATP molecules are used to phosphorylate glucose - this makes the molecule unstable - “phosphorylation”
The phosphorylated glucose is split into 2 G3P (triose phosphate) molecules “lysis”
Glycolysis: Energy payoff phase
Electrons and hydrogens are removed from the 2 G3P molecules - “oxidation” and “dehydrogenation”
The electrons (and hydrogens) are then transferred to 2 NAD+ to make 2 NADH
NAD+/NADH (Nicotinamide Adenine Dinucleotide) is an electron carrier
NAD+ is the oxidized state (“empty”)
NADH is the reduced state (“full”)
4 ATP are produced using substrate level phosphorylation - “ATP formation”
Substrate level phosphorylation - a metabolic reaction that results in the formation of ATP by the direct transfer of a phosphate group to ADP from another phosphorylated compound
The enzyme takes the phosphate group from its substrate and attaches it to ADP to make ATP (phosphorylation)
During the energy payoff phase, the 2 G3P are converted into 2 pyruvate
Glycolysis: Overall
Glucose → 2 pyruvate
Also produced:
2 NADH
4 ATP (net 2 ATP)
After Glycolysis
If O2 is present: pyruvate will enter the mitochondria
After entering the mitochondria, the following stages will occur:
Link reaction
Kreb cycle
OxPhos
Link reaction
Occurs in the Mitochondrial matrix
Maintains a low concentration of pyruvate in the Mitochondrial Matrix
2 things are removed from pyruvate after it enters the matrix:
CO2 (decarboxylation)
Electrons (oxidation)
“Oxidative decarboxylation”
After oxidative decarboxylation, Coenzyme A will be added
produces 1 Acetyl-CoA per pyruvate
Net products (glycolysis + link)
For one glucose molecule we have:
2 Acetyl-CoA
2 CO2 (from link reaction)
2 ATP (from glycolysis)
4 NADH (2 from glycolysis, 2 from link reaction)
Krebs cycle
Occurs in the mitochondrial matrix in the presence of O2
A cyclical metabolic pathway made of 8 reactions each with their own enzyme
Finishes the breakdown of glucose
Acetyl-CoA (2C) is combined with oxaloacetate (4C) to release the Coenzyme-A and make citrate (6C)
The Krebs cycle is also called the Citric ACid cycle after citrate (citric acid)
Citrate then undergoes oxidative decarboxylation to produce:
NADH
CO2
5-Carbon compound
The 5-Carbon compound undergoes oxidative decarboxylation, producing:
NADH + CO2
4-Carbon compound
An ATP is also made by substrate level phosphorylation
Remember: this is a cycle - the starting molecule must be regenerated
The 4-carbon compound is converted back into oxaloacetate
Produces NADH and FADH2 in the process
FAD/FADH2 (Flavin Adenine Dinucleotide) is an electron carrier
FAD is the oxidized state (“empty”)
FADH2 is the reduced state (“full”)
FADH2 can carry 1 more hydrogen compared to NADH
Krebs cycle products
2 Acetyl-CoA enter to produce:
4 Co2
6 NADH
2 FADH2
2 ATP
Remember: The Krebs cycle finishes the breakdown of glucose (and it “fills” the largest number of electron carries)
Oxidative Phosphorylation (OxPhos)
OxPhos creates the largest amount of ATP during the cellular respiration process
Utilizes O2 - Aerobic
OxPhos is accomplished by utilizing an electron transport chain and ATP synthase
OxPhos: Electron transport Chain
The Mitochondrial ETC is made of a series of protein complexes that are embedded in the inner mitochondrial membrane
Cristae increase the surface area of the inner mitochondrial membrane
NADH and FADH2 will drop off their electrons with the ETC creating NAD+ and FAD
As electrons are transferred the ETC pumps H+ (protons) into the intermembrane space - creates a proton concentration gradient
O2 is the final electron acceptor of the mitochondrial ETC
O2 combines with the electrons and will pick up protons (H+) from the mitochondrial matrix - this creates H2O
OxPhos is Aerobis because O2 is required for the process to happen.
A lack of O2 would cause a backup of electrons in the ETC and would shut the process down
Mitochondrial proton gradient
High concentration of H+ in the intermembrane space
Low concentration of H+ in the mitochondrial matrix
Intermembrane Space:
High concentration of H+ in the intermembrane space because:
ETC pumps H+ into the intermembrane space
O2 combines with H+ to make water in the matrix, reducing the H+ concentration
Intermembrane space is small
OxPhos: Chemiosmosis
The proton concentration gradient allows for passive transport out of the intermembrane space (down its concentration gradient)
Can the protons (H+) pass through the membrane unassisted via simple diffusion?
NO!
Because of their charge, protons (H+) can only exit the intermembrane space via a transmembrane integral protein
Chemiosmosis is the diffusion of H+ down its concentration gradient through ATP synthase
OxPhos: Chemosmosis & ATP Synthase
Transmembrane integral protein that is also an enzyme (-ase_
ATP synthase performs ADP phosphorylation to create (synthesize) ATP
This process requires energy
OxPhos: Chemiosmosis
As the H+ diffuses through ATP synthase, it causes the enzyme to turn - much like a water wheel creating power
This provides the energy needed to phosphorylate ADP into ATP
OxPhos:ATP production
Most of the ATP produced during cellular respiration is produced during the OxPhos step
A theoretical maximum of 32-34 ATP can be produced during OxPhos
ATP Production Overall
Glycolysis = 2 ATP (net)
Link reaction = 0 ATP
Krebs cycle = 2 ATP
OxPhos = up to 34 ATP
Fermentation (Anaerobic respiration)
Vocabulary:
Pyruvate -
Fermentation -
Generating ATP with O2
Glycolysis occurs in the cytoplasm of a cell
When O2 is present - pyruvate will enter the mitochondria and the following processes will occur:
Link Reaction
Krebs Cycle
OxPhos
Generating ATP without O2
Glycolysis occurs in the cytoplasm
When there is no O2 present, pyruvate will stay in the cytoplasm and enter:
Alcohol Fermentation
OR
Lactic Acid Fermentation
Glycolysis review
Converts glucose into 2 pyruvate molecules
Produces 2 ATP (net) and 2 NADH
Glycolysis requires NAD+ and ADP to continue
Making NAD+ and ADP available (with O2)
When oxygen is present:
NADH will drop off their electrons with the ETC: Creating NAD+
ATP is continually used for various cellular functions: Creates ADP
Without O2 present, NADH cannot drop off their electrons with the ETC
Without O2: Glycolysis Couples with fermentation
Fermentation regenerates NAD+ in order to allow glycolysis to continue under anaerobic conditions:
Occurs in the cytoplasm - all cells can do fermentation
2 types:
Alcohol fermentation
Lactic acid fermentation
Glycolysis is how ATP is created in anaerobic conditions (net 2 ATP)
Alcohol fermentation
1st Glycolysis:
Glucose →
2 Pyruvate + 2 ATP + 2 NADH
Then:
2 Pyruvate + 2 NADH →
2 Ethanol + 2 CO2 + 2 NAD+
Overall:
Glucose →
2 Ethanol + 2 CO2 + 2 ATP
Yeast (ex. Saccharomyces cerevisiae) and zymomonas mobilis (an anaerobic bacterium)
Uses in industry:
Baking bread
Alcoholic beverages
Also called alcoholic fermentation or ethanol fermentation
Lactic Acid Fermentation
1st Glycolysis:
Glucose →
2 Pyruvate + 2 ATP + 2 NADH
Then:
2 Pyruvate + 2 NADH →
2 Lactate + 2 NAD+
Overall:
Glucose → 2 Lactate + 2 ATP
Humans, Animals, and some Bacteria (ex. from the genera Escherichia, and Lactobacillus)
Uses in industry (not exhaustive):
Yogurt
Kimchi
Cheese
Pickles
Lactic Acid Fermentation in Humans
Humans have large energy (ATP) requirements, so lactic acid fermentation is not a good long-term solution for us because only 2 ATP (net) are produced.
However, it is effective for generating ATP for short, intense exercises (ex. Sprinting or weightlifting)
Pros and Cons of Anaerobic Respiration
Pros:
Produces ATP in the absence of O2
Faster
Doesn’t require special structures (ETC/ATP synthase)
Cons:
Much less ATP than aerobic
Pros and cons of Aerobic respiration
Pros:
Much more ATP is produced
Cons:
Requires O2
Requires special structures (ETC/ATP synthase)
Slower
Practice Questions
ATP
What is the role of ATP in plants?
How is ATP produced in plants?
Which cellular processes in plants require ATP?
Can plants store ATP for later use?
How is ATP used in photosynthesis?
What happens to ATP during cellular respiration in plants?
Are there any specific enzymes involved in ATP synthesis in plants?
How does ATP contribute to plant growth and development?
Can plants obtain ATP from sources other than cellular respiration?
Are there any factors that can affect ATP production in plants?
What is the process of converting ATP to ADP?
What enzyme is responsible for the conversion of ATP to ADP?
How is the conversion of ATP to ADP related to energy release?
Can ADP be converted back to ATP in plants?
What role does ADP play in cellular metabolism?
Are there any specific cellular processes that require ADP?
How does the conversion of ATP to ADP affect plant growth and development?
Can plants obtain ADP from external sources?
Are there any factors that can affect the conversion of ATP to ADP in plants?
How does the ratio of ATP to ADP impact cellular energy levels in plants?