Globular oxygen binding heme-proteins
Free iron can participate in Fenton reactions, generating reactive oxygen species (ROS) that can damage cellular components and it cannot bind oxygen
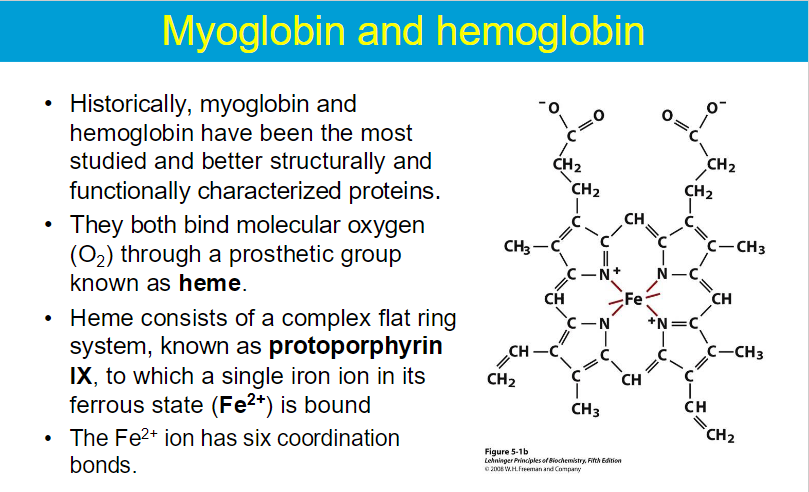
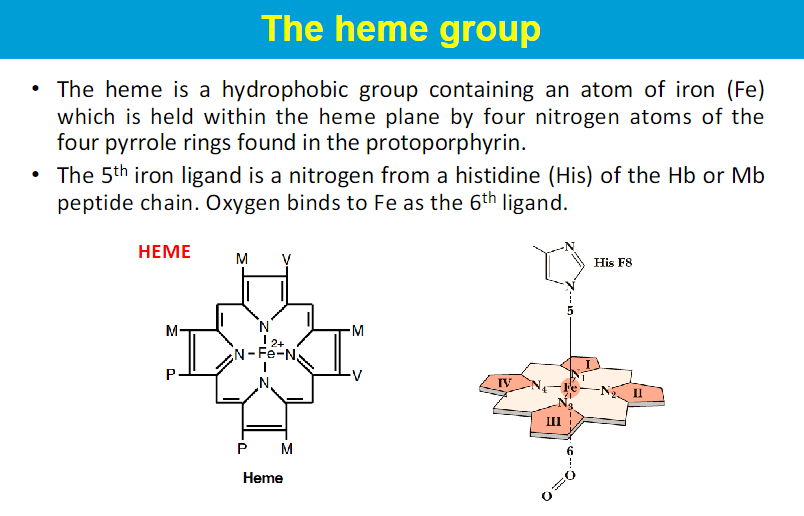
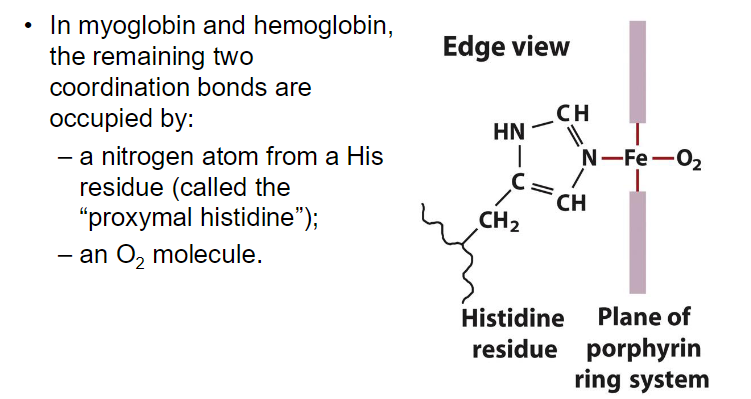
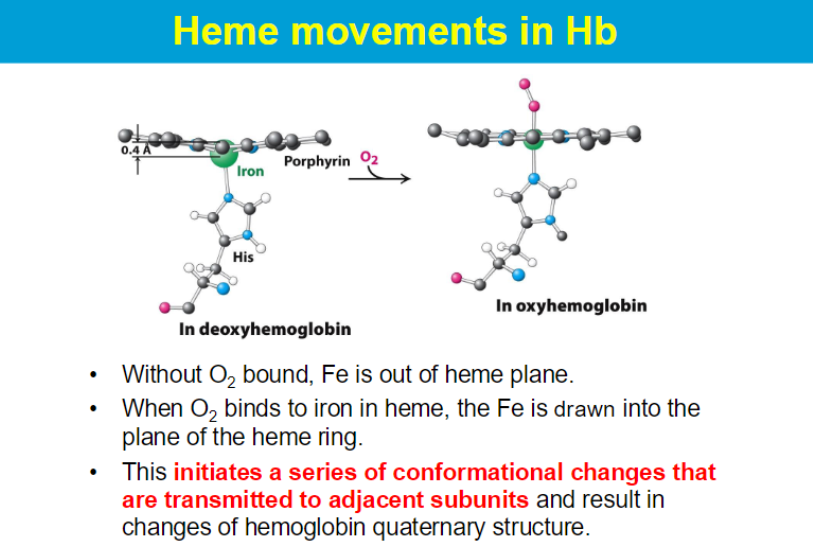
The heme group is embedded within the protein structure, providing a stable environment that prevents the oxidation of Fe²⁺ to Fe³⁺.
Myoglobin is primarily a storage protein found in muscle tissues.
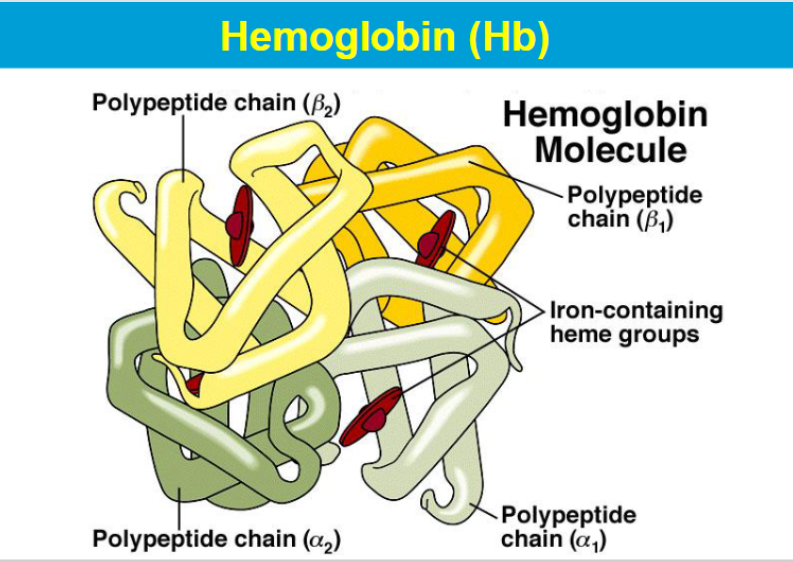
Hemoglobin, found in red blood cells, is specialized for the transport of oxygen.
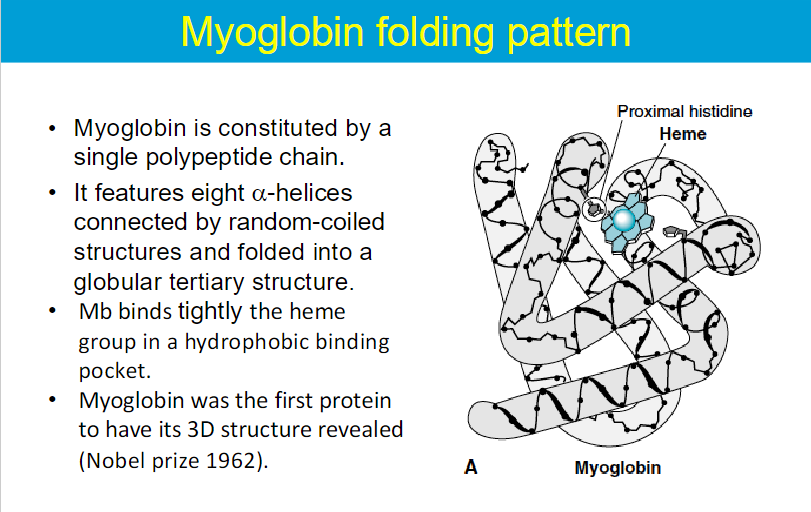
Myoglobin vs Hemoglobin
Oxygen binds protein in muscles and RBC
Myoglobin will only give up oxygen at very low oxygen partial pressures.
Oxygen bound myoglobin (oxy-Mb) is much more stable than oxygen bound hemoglobin.
Hemoglobin transports oxygen to tissues.
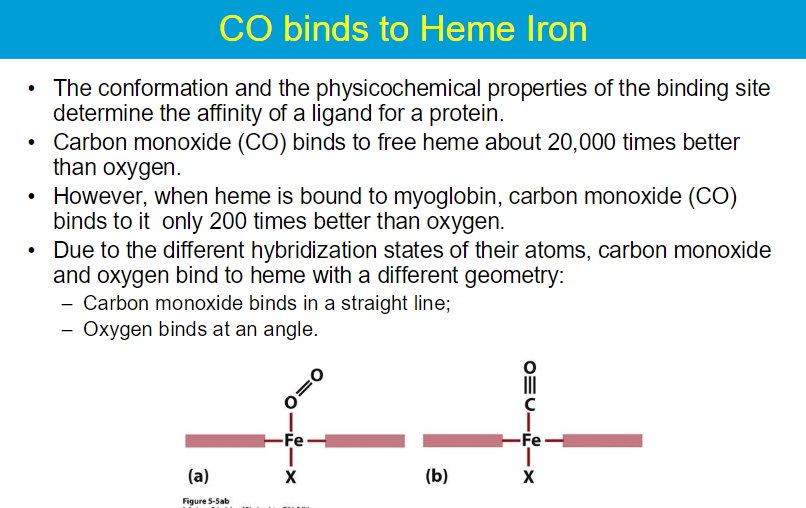
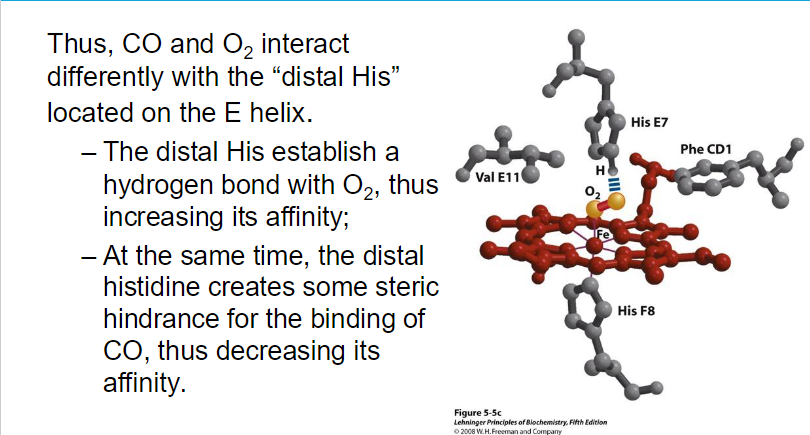
**While myoglobin can bind oxygen at higher pO₂ levels, its high affinity results in poor release at lower pO₂, making it primarily a storage protein.
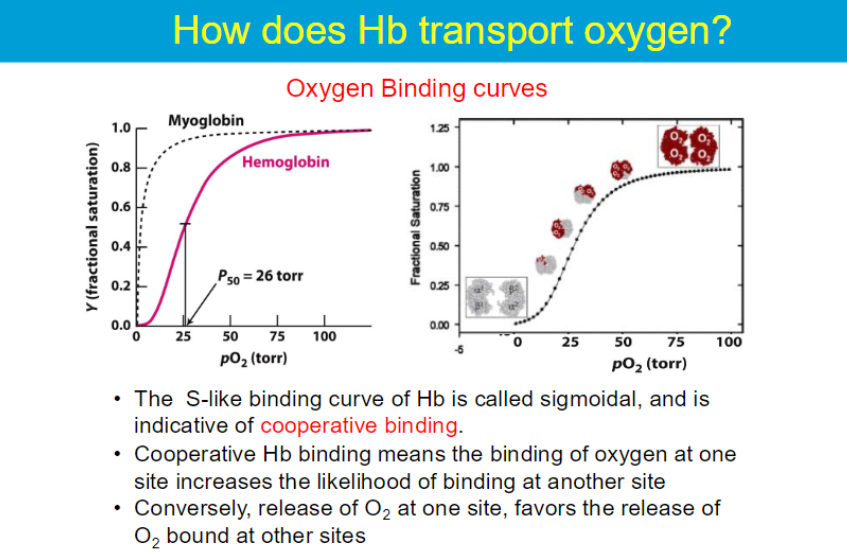
sigmoidal means that at low oxygen concentrations, hemoglobin's affinity for oxygen is lower, but as oxygen concentration increases, the affinity increases significantly.
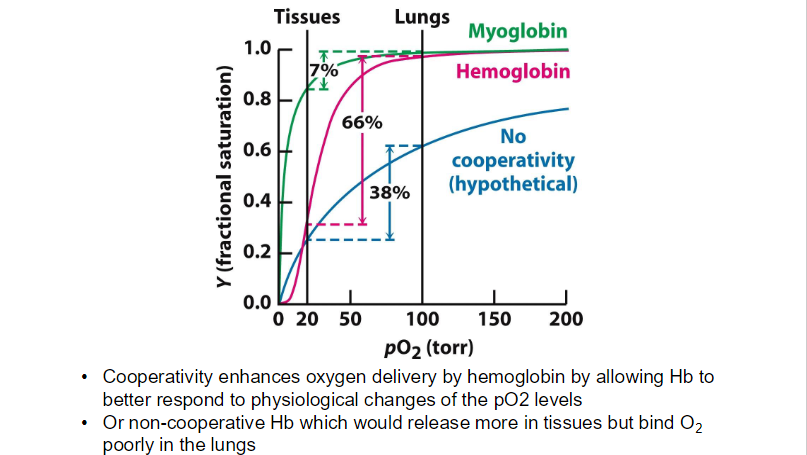
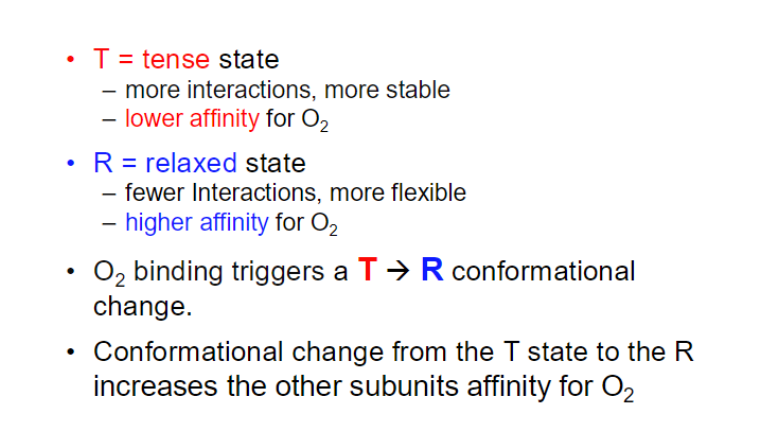
The high pO₂ in the lungs promotes the transition of hemoglobin to a "relaxed" (R) state, which has a higher affinity for oxygen.
The drop in pO₂ in tissuses causes hemoglobin to transition to a "tense" (T) state, which has a lower affinity for oxygen.
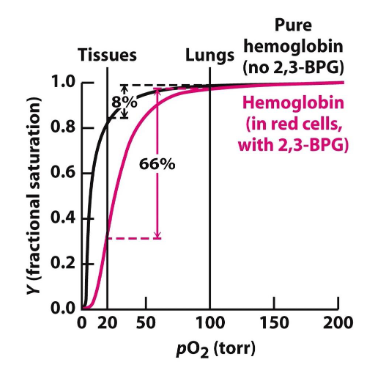
when Hb is purified → has similar activity to Mb (high affinity to O2)
the presence of 2,3-BPG in RBCs stabilizes the T state of hemoglobin, facilitating oxygen release in low pO₂ environments.
has a 1:1 ratio with Hb in RBCs
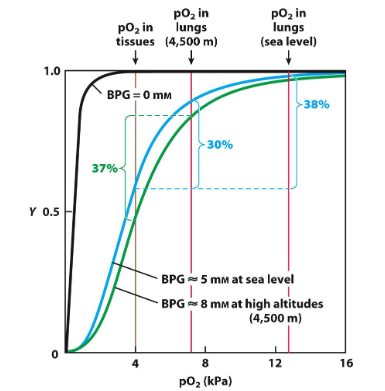
When individuals ascend to high altitudes, the partial pressure of oxygen (pO₂) decreases —> the body responds by increasing the concentration of 2,3-bisphosphoglycerate (2,3-BPG) in red blood cells.
The increase in 2,3-BPG levels leads to a decrease in hemoglobin’s affinity for oxygen. This is represented by a rightward shift in the oxygen-binding curve
greater effect in tissues than in the lungs
increased levels of 2,3-BPG help hemoglobin to release oxygen more effectively to tissues. As you noted, hemoglobin releases about 37% of the bound oxygen at high altitudes, similar to what would occur at sea level.
In the absence of the 2,3-BPG effect, the affinity of hemoglobin for oxygen would remain higher, leading to a reduced release of oxygen to tissues. Without this adaptation, only about 30% of the bound oxygen would be released at high altitudes.
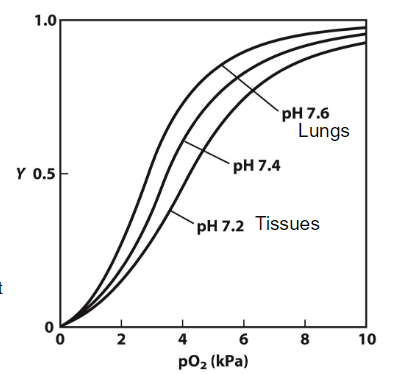
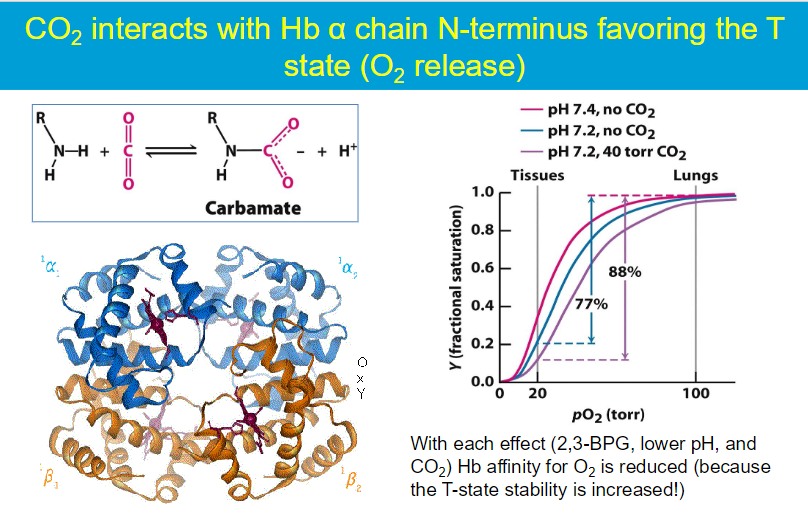
The Bohr effect refers to the inverse relationship between the pH of the blood and the affinity of hemoglobin for oxygen. As pH decreases (more acidic), the affinity of hemoglobin for oxygen decreases, leading to a higher P50 (the partial pressure of oxygen at which hemoglobin is 50% saturated)
higher P50 = lower affinity to O2
mechanism: At lower pH levels, protons (H⁺) bind to specific histidine residues in hemoglobin. This binding stabilizes the T (tense) state of hemoglobin, which has a lower affinity for oxygen.
tissues: the production of carbon dioxide (CO₂) from cellular respiration lowers the pH of the blood (due to carbonic acid formation)
lungs: When hemoglobin reaches the lungs, the pH is relatively higher (more alkaline) due to lower CO₂ concentrations and the conversion of carbonic acid to bicarbonate.
This higher pH promotes the release of protons that were bound to hemoglobin in the tissues → hemoglobin shifts to the R (relaxed) state, which has a higher affinity for oxygen. This means that hemoglobin can effectively bind oxygen in the lungs where the pO₂ is high.
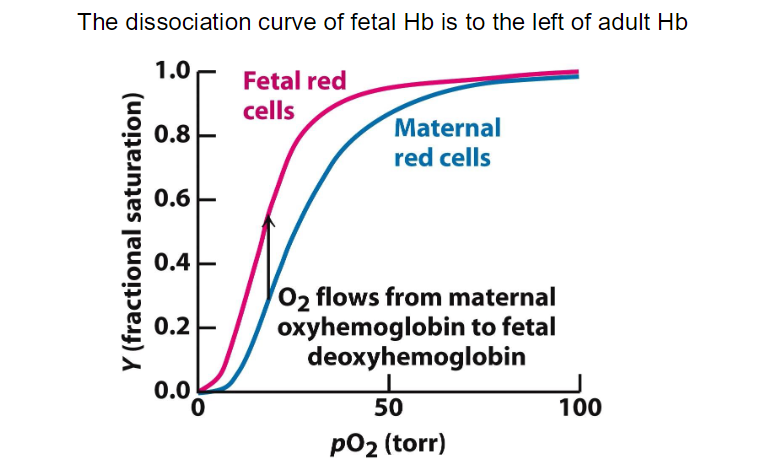
**the developing fetus relies on maternal oxygen through a highly efficient exchange mechanism at the placenta. Fetal hemoglobin's higher affinity for oxygen ensures that it can effectively extract oxygen from maternal blood, supporting the growth and development of the fetus.