TOPIC 10 - USING RESOURCES
10.1 Using the Earth’s Resources and Obtaining Potable Water
Using the Earth’s Resources and Sustainable Development
We use Earth’s resources to provide warmth, shelter, food, and transport.
Natural resources, supplemented by agriculture, provide food, timber, clothing, and fuels.
Finite resources from the Earth, oceans, and atmosphere are processed to provide energy and materials.
Chemistry plays an important role in improving agricultural and industrial processes to provide new products and in sustainable development, which meets the needs of current generations without compromising the ability of future generations to meet their own needs.
Renewable Energy Resources → Sources of power that quickly replenish themselves and can be used again (only includes plants/wood if they continue to be replanted).
Finite Resources → Have a limited supply that will eventually run out.
Potable Water
Potable Water → Water that is safe to drink.
Potable water is not ‘pure’ because it contains dissolved substances; although to be safe, it must have sufficiently low levels of dissolved salts and microbes.
The methods used to produce potable water depend on available supplies of water and local conditions.
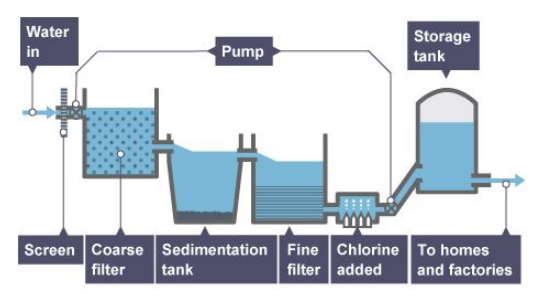
In the UK:
An appropriate source of fresh water is selected.
Rain provides water with low levels of dissolved substances and this collects in the ground, rivers, or lakes.
The water is passed through filter beds to remove different-sized insoluble solids.
The water is then sterilized to kill microbes.
Sterilizing agents include ozone, UV light, or chlorine.
If only salty/sea water is available, desalination is required:
Can be done by distillation.
Or it can be done using processes with membranes (e.g. reverse osmosis).
Both are very expensive because they require a large amount of energy.
Wastewater Treatment
Water of the correct quality is essential for life. It must be free of poisonous salts and harmful microbes.
How correct quality water is produced:
Water is passed through a mesh screen to remove large bits (e.g. twigs or grit).
Chemicals are added to make solids and microbes stick together to form sediment and sink.
There is then anaerobic digestion of sewage sludge.
The effluent is usually treated using aerobic biological treatment.
The water is then sterilized with chlorine to kill any microbes left.
It is relatively cheaper and easier to obtain potable water from groundwater and wastewater than salt water, although seawater is a plentiful raw material, so is good for countries with little freshwater.
Alternative Methods of Extracting Metals
Earth’s resources of metal ores are limited.
Copper ores are becoming scarce and new ways of extracting copper from low-grade ores include phytomining and bioleaching.
These methods avoid traditional mining methods of digging, moving, and disposing of large amounts of rocks.
Phytomining → Uses plants to absorb metal compounds.
Plants are harvested and then burned to produce ash that contains metal compounds.
Bioleaching → Uses bacteria to produce leachate solutions that contain metal compounds.
The metal compounds can be processed to obtain the metal.
For example, copper can be obtained from solutions of copper compounds by displacement using scrap iron or by electrolysis.
10.2 Life Cycle Assessment and Recycling
Life Cycle Assessment
Life cycle assessments(LCAs) are carried out to assess the environmental impact of products in each of these stages:
Extracting and processing raw materials
Manufacturing and packaging
Use and operation during its lifetime
Disposal at the end of its useful life, including transport and distribution at each stage
Use of water, resources, energy sources, and production of some wastes can be fairly easily quantified.
Allocating numerical values to pollutant effects is less straightforward and requires value judgements, so LCA is not a purely objective process.
Selective or abbreviated LCAs can be devised to evaluate a product but these can be misused (e.g. in support of claims for advertising purposes).
Ways of Reducing the Use of Resources
Reduction in use, reuse, and recycling of materials by end users reduces the use of limited resources, use of energy sources, waste, and environmental impacts.
Metals, glass, building materials, clay ceramics, and most plastics are produced from limited raw materials.
Much of the energy for the processes comes from limited resources.
Obtaining raw materials from the Earth by quarrying and mining causes environmental impacts.
Some products, such as glass bottles, can be reused.
Glass bottles can be crushed and melted to make different glass products.
Other products cannot be reused and so are recycled for a different use.
Metals can be recycled by melting and recasting or reforming into different products.
The amount of separation required for recycling depends on the material and the properties required of the final product.
E.g. Some scrap steel can be added to iron from a blast furnace to reduce the amount of iron that needs to be extracted from iron ore.
10.3 Using Materials
Corrosion and Its Prevention
Corrosion → Destruction of materials by chemical reactions with substances in the environment.
E.g. Rusting
Both air and water are necessary for iron to rust.
Corrosion can be prevented by applying a coating that acts as a barrier, such as greasing, painting, or electroplating.
Aluminum has an oxide coating that protects the metal from further corrosion.
Some coatings are reactive and contain a more reactive metal to provide sacrificial protection.
E.g. Zinc is used to galvanize iron
Sacrificial protection works by having the more reactive metal donate electrons to any ions of the other metal that may have formed, preventing the other metal from corroding.
Alloys as Useful Materials
Most metals in everyday uses are alloys. Pure copper, gold, iron, and aluminum are too soft for everyday uses and so are mixed with small amounts of similar metals to make them harder for everyday use.
Gold in jewelry is usually an alloy with silver, copper, and zinc. Gold purity is measured in carats.
Bronze is an alloy of copper and tin, which is used in electrical connectors.
Brass is an alloy of copper and zinc, which is used for tools.
Steels are alloys since they use mixtures of carbon and iron.
Some steels contain other metals. Alloys can be designed for specific uses:
Low-carbon steels are easily shaped. It is commonly used for sheeting due to its malleability.
High-carbon steels are hard but usually brittle. It is commonly used for cutting tools.
Stainless steels (usually containing chromium and nickel), are resistant to corrosion and are used for cutlery.
Aluminum alloys have low density and are used for aircraft and automotive.
Ceramics, Polymers, and Composites
Soda-lime Glass → Made by heating a mixture of sand, sodium carbonate, and limestone (most commonly used glass).
Borosilicate Glass → Made from sand and boron trioxide, melts at higher temperatures than soda-lime glass.
Clay Ceramics (pottery and bricks) → Made by shaping wet clay and then heating in a furnace.
Properties of polymers depend on what monomers they are made from and the conditions under which they are made.
E.g Low density polyethylene and high density polyethylene (both produced from ethene) are made under different reaction conditions using different catalysts.
Low density polyethylene has weaker forces of attraction, as the chains are further apart due to its structure, meaning it has a low melting point and is soft.
High density polyethylene has higher forces of attraction, as the chains are closer together due to its structure, giving it a higher melting point.
Thermosoftening polymers are individual, tangled polymer chains which are easily separated and melt when heated.
There are weak intermolecular forces between the chains.
The chains are easy to separate at lower temperatures.
Less heat energy is needed to break the chains.
Thermosetting polymers consist of polymer chains, which have cross-links, so that they do not melt when heated.
Most composites are made of two materials, a matrix or binder surrounding and binding together fibers or fragments of the other material, which is called the reinforcement.
Examples of composites: Carbon fiber and fiberglass—both are very hard but very brittle.
10.4 The Haber Process and the Use of NPK Fertilizers
The Haber Process
Used to manufacture ammonia, which is used to produce nitrogen-based fertilizers.
The raw materials are nitrogen and hydrogen.
Nitrogen is obtained from the air and hydrogen may be obtained from natural gas or other sources.
The purified gases are passed over a catalyst of iron at a high temperature (about 450 °C) and a high pressure (about 200 atmospheres).
Some of the hydrogen and nitrogen reacts to form ammonia.
The reaction is reversible so ammonia breaks down again into nitrogen and hydrogen.
Nitrogen + Hydrogen ⇋ Ammonia
On cooling, the ammonia liquefies and is removed.
The remaining nitrogen and hydrogen are recycled.
Reaction conditions and compromise:
The Haber Process is in dynamic equilibrium—the forward and backward reactions keep going once equilibrium is reached.
The chemical equation is N2 + 3H2 ⇋ 2NH3, so there are less moles of gas on the product side (2 compared to 4), this means you would increase pressure to move equilibrium to the right so more ammonia is produced.
The forward reaction is exothermic, so a low temperature would favor the forward reaction and mean more ammonia was produced.
However, the actual conditions used are not low temperature and very high pressure because a low temperature leads to a reaction rate that is too slow and a very high pressure requires too much energy.
Therefore an optimal temperature of 450 °C and a pressure of 200 atmospheres are used to maximize the yield of ammonia, while also maintaining a reasonable reaction rate and reducing energy usage.
Production and Uses of NPK Fertilizers
Compounds of nitrogen, phosphorus, and potassium are used as fertilizers to improve agricultural productivity.
NPK fertilizers contain compounds of all three elements.
Industrial production of NPK fertilizers can be achieved using a variety of raw materials in several integrated processes.
NPK fertilizers are formulations of various salts containing appropriate percentages of the elements.
Ammonia can be used to manufacture ammonium salts and nitric acid.
Potassium chloride, potassium sulfate, and phosphate rock are obtained by mining, but phosphate rock cannot be used directly as a fertilizer.
Phosphate rock is treated with nitric acid or sulfuric acid to produce soluble salts that can be used as NPK fertilizers.