Carbohydrates and Glycoconiugates
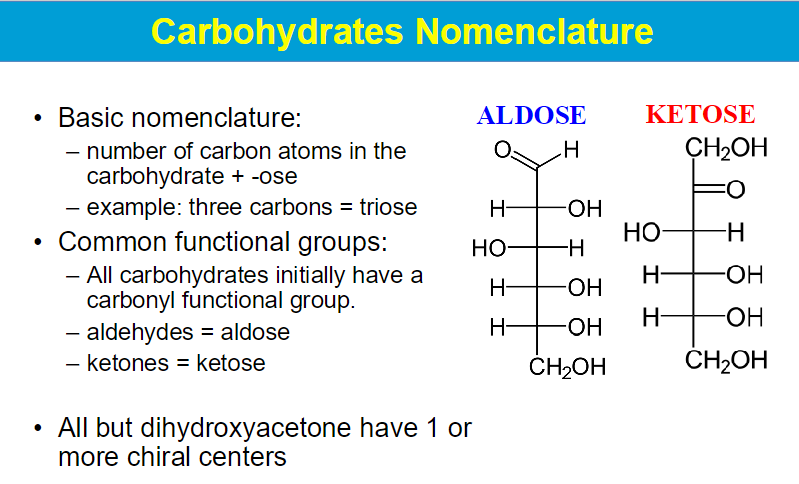
all C are chiral except the C in position 1 (is this for aldehydes lol
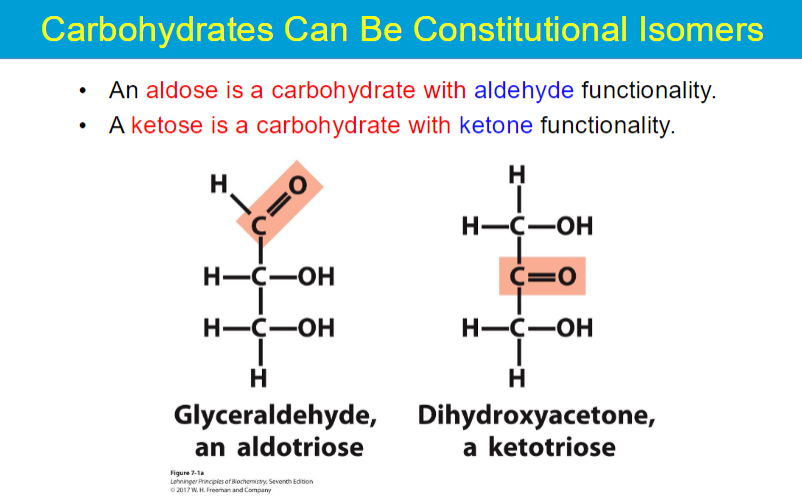
minimum 3 to make a glyceraldehyde ???? what does that mean bruh
dihydroxyacetone has no chiral centers??
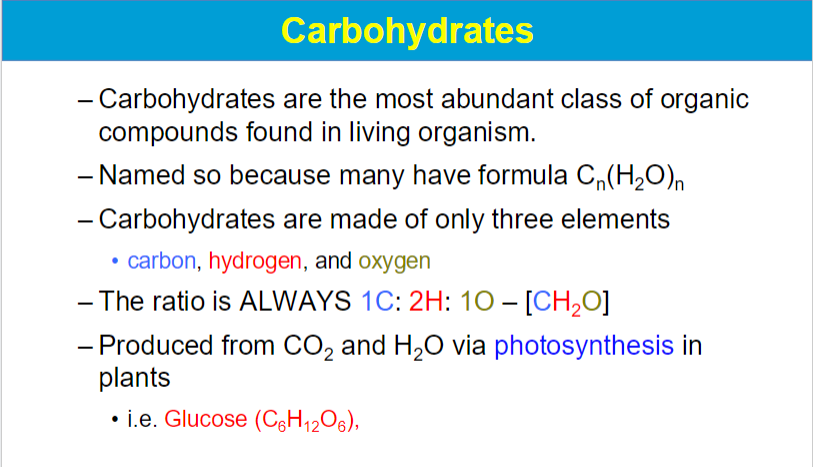
possible to have very large carbohydrates
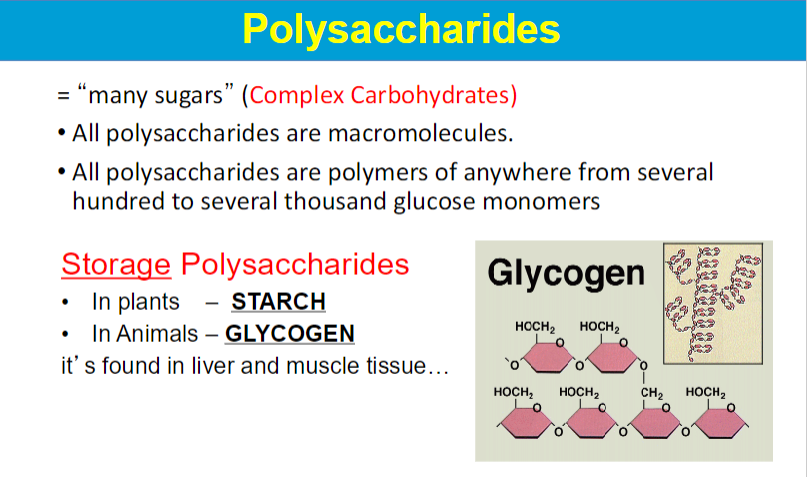
**carbs are main source of energy for body; main ways to store energy
lipids also store energy
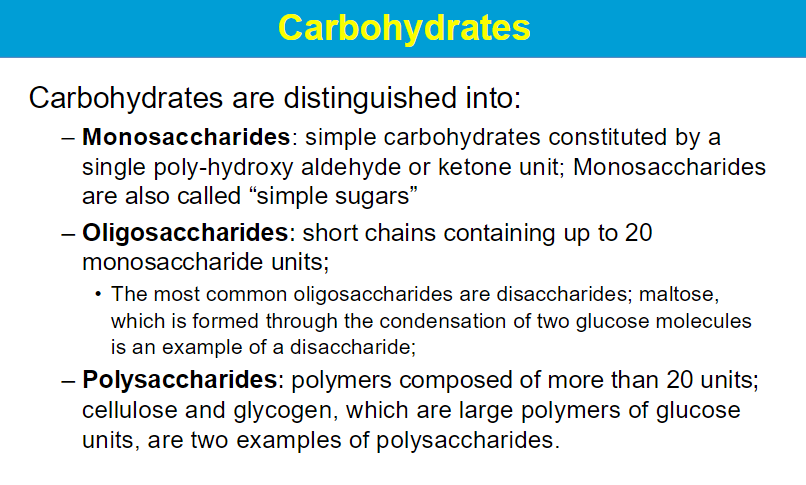
mono - 1 unit that does not repeat
disaccharide - 2 units connected
poly - long chain w repeats of the same units
idk he said something about glucose connects in diffeent way???
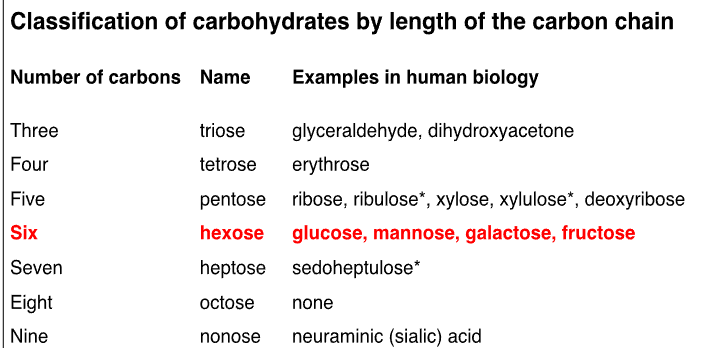
**hexose most important to us ; biggest group
**pentose also→ ex. ribose contained in DNA and RNA
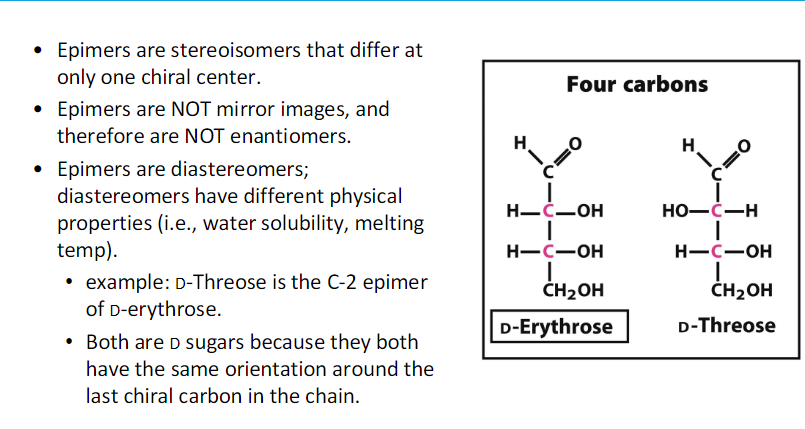
stereoisomers !!

^^ mirror images of each other
ex.
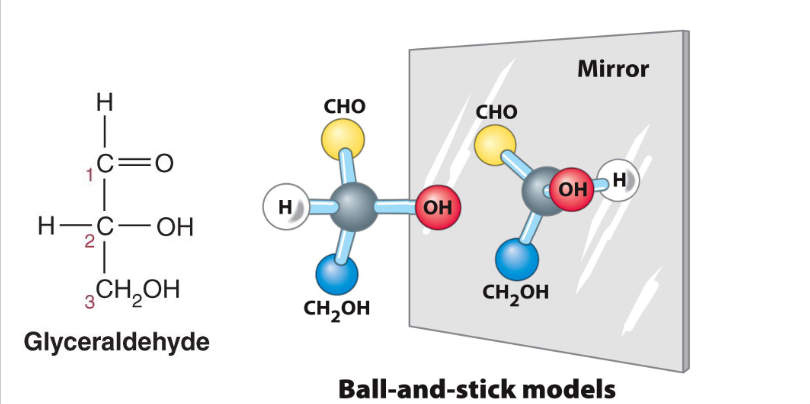
fischer projection
vertical parts are going into the paper
horizontal parts are pointing towards you
D- glyceraldehyde: O group is on the right
L - glyceraldehyde: O group on the left
more than one chiral C and then what lol??

Common Carbohydrates in Biochemistry
Ribose is the standard five-carbon sugar.
Glucose is the standard six-carbon sugar.
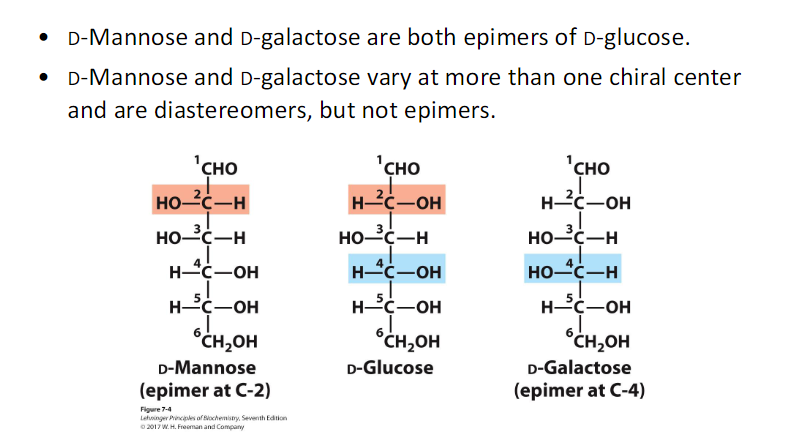
Galactose is an epimer of glucose.
Mannose is an epimer of glucose.
Fructose is the ketose form of glucose.
Lactose (milk sugar) is the disaccharide found in milk.
**memorize structure of glucose (linear structure)
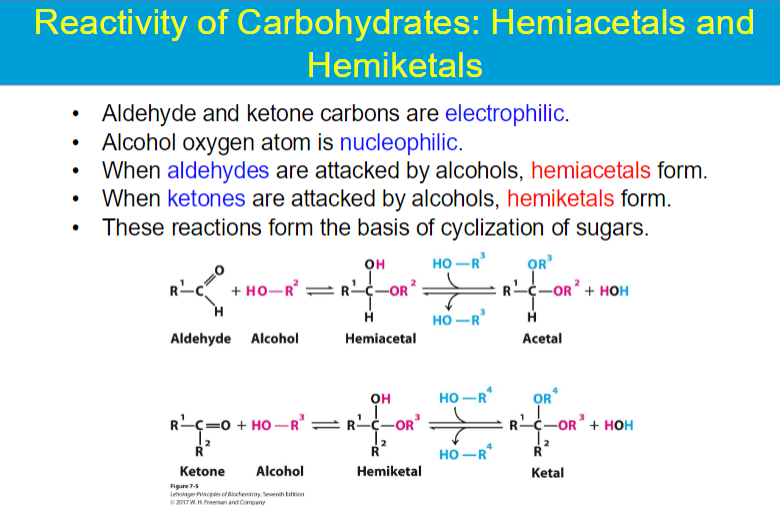
alcohol (OH) of C5 attacks the C (1) of C=O (aldehyde or ketone)
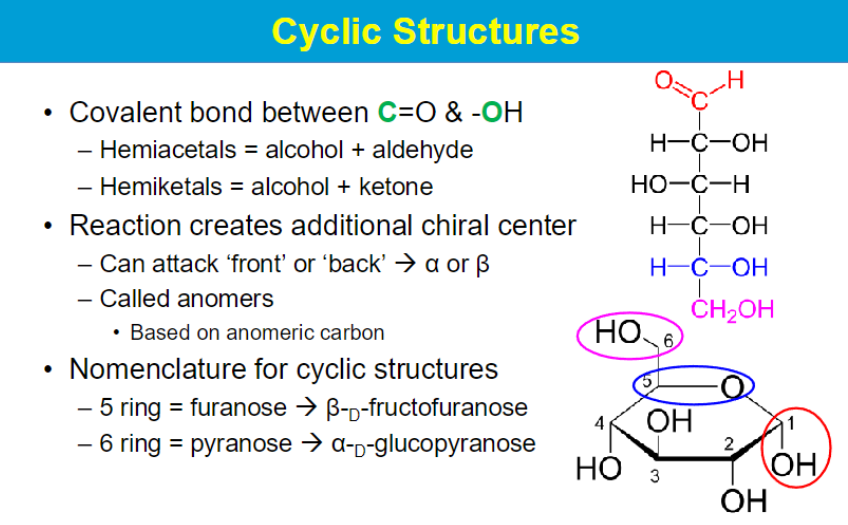

anomeric carbon = C1 → depends on if the attack is front or back
OH in axial or equatorial position
axial = alpha (front attack) ; equatorial = beta (back attack)
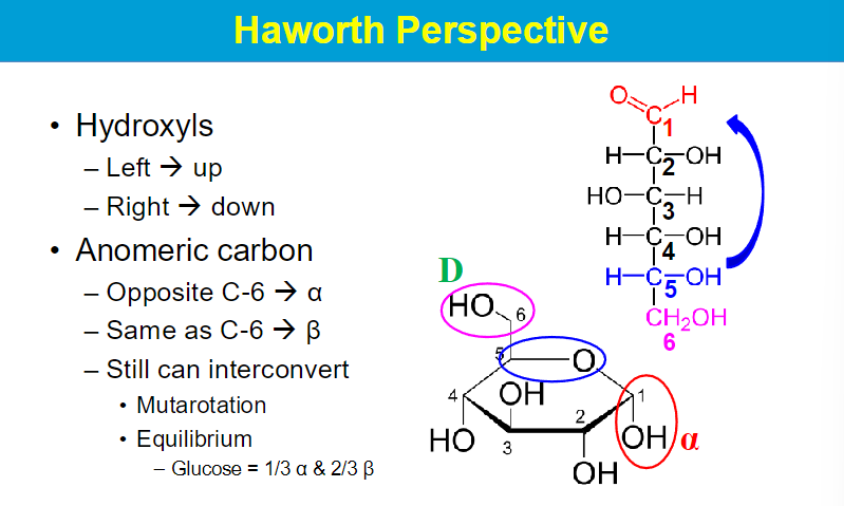
can interconvert? between alpha and beta cia mutarotation
alpha: OH of anomeric carbon (1) is trans to the OH of C6
beta: same side as CH2OH molecule → cis

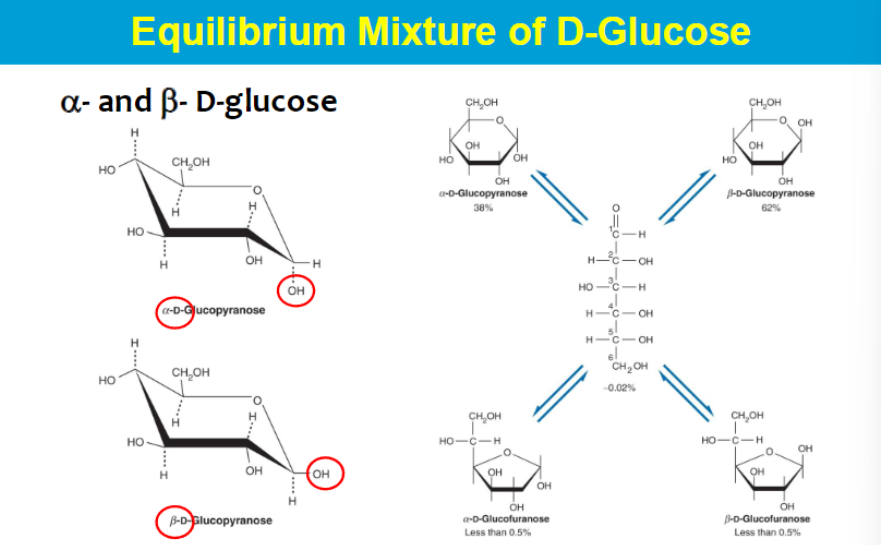
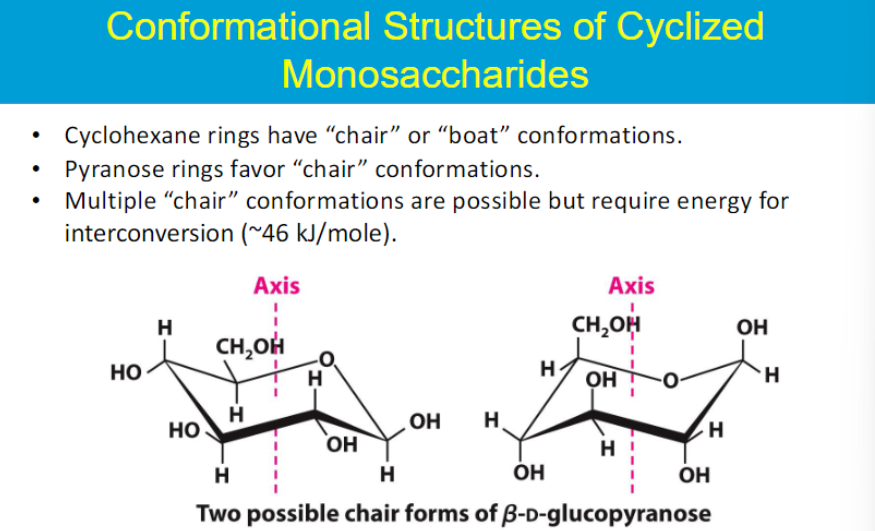
2 possible chair conformations (bc of alpha and beta?)
interconversion between the two but need some energy to move from one to another
they are in equilibrium but usually 1 is prefered
derivation of glucose
If a substance oxidizes, it combines with oxygen and loses hydrogen to form another substance, (loses electrons) (OIL RIG)
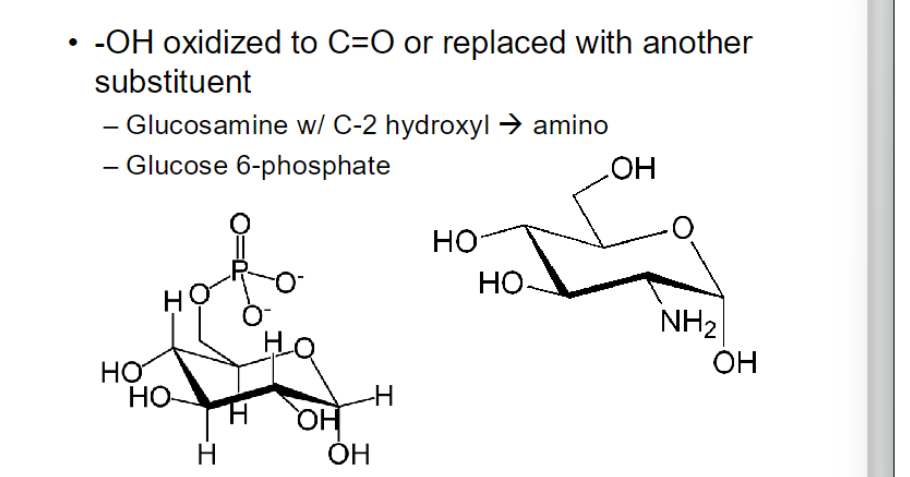
glucose phosphorylated at position 6 → glucose 6-phosphate
reducing agents lose electrons (are oxidized) to reduce another molecule
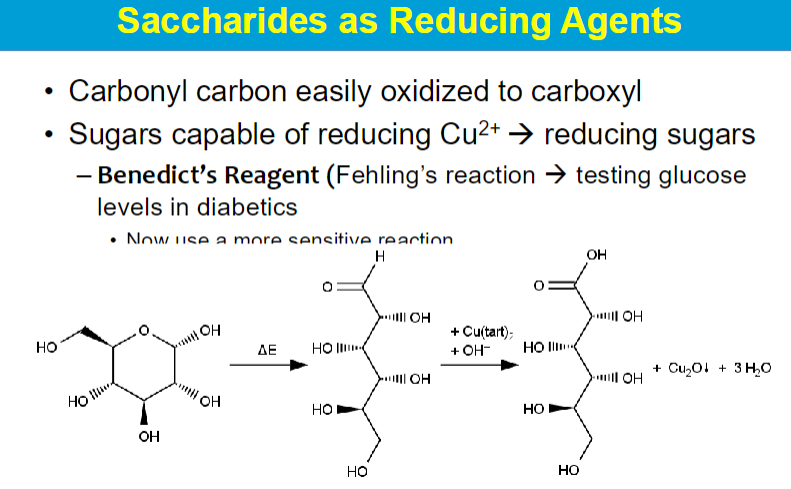
sugars can be oxidized: carbonyl → carboxyl
cyclic form opens up → carbonyl → carboxyl (oxidized to respective acid)
fehling’s reaction
cu2+ is an agent that can be reduced by sugar (reducing agent)
copper precipitate: visual way to test sugar
test urine for diabetes (test for glucose)
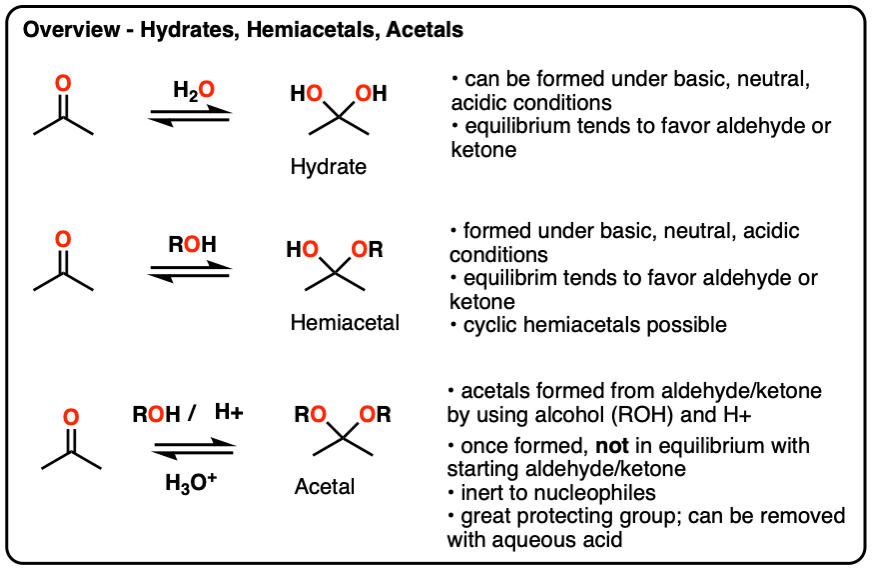
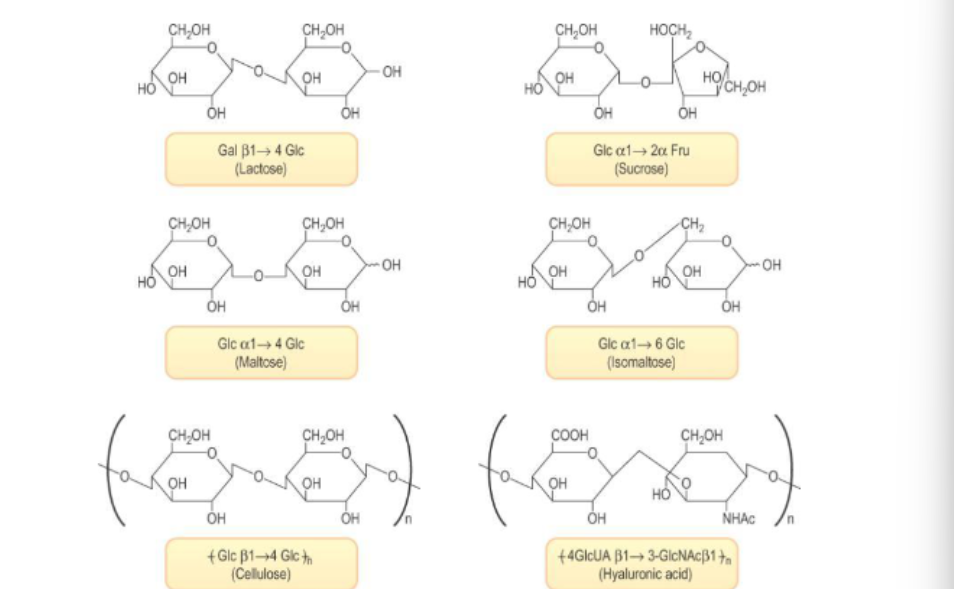
di-saccharides
bind together 2 monosaccharides w covalent bond
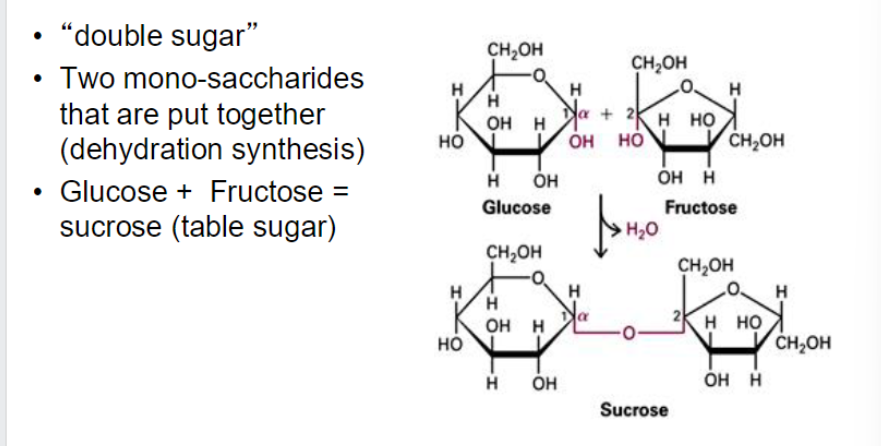
molecule of water is removed
sucrose forms alpha bond between position 1 and position 2 ???
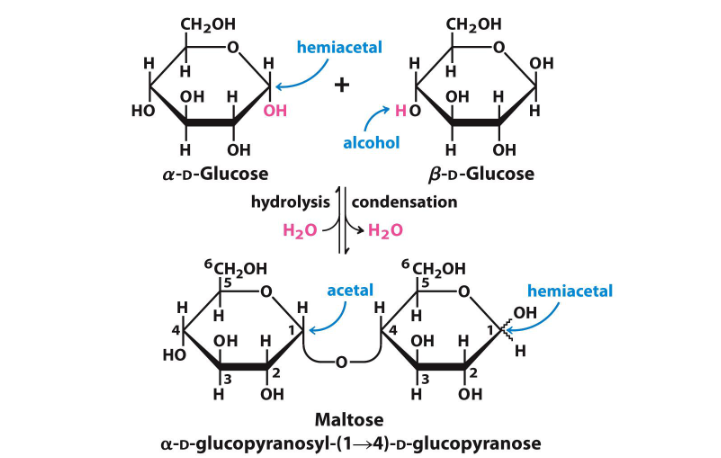
The second sugar, which retains a hemiacetal structure (meaning it has a free anomeric carbon), is more reactive. Hemiacetals can easily interconvert between their cyclic and open-chain forms, allowing them to participate in further reactions, such as oxidation or reduction.
In contrast, once the anomeric carbon is involved in a glycosidic bond, it becomes part of a more stable acetal structure and is less reactive.
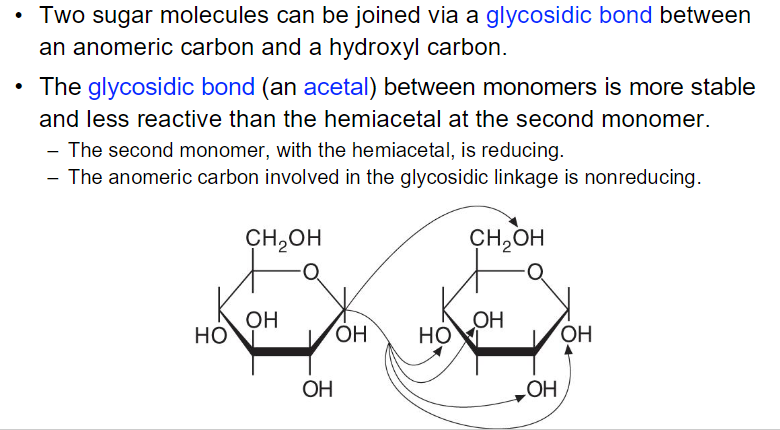
^ what does the picture mean?? like any of these bonds can form?
Naming Disaccharides
Identify the Anomeric Carbon:
In maltose, the glycosidic bond is formed between the anomeric carbon of the first glucose (α-D-glucopyranose) and the hydroxyl group on the fourth carbon of the second glucose. Since the first glucose is in the α configuration, the bond is designated as α.
Nonreducing Residue:
The first glucose unit, which is involved in the glycosidic bond and is nonreducing, is named as α-D-glucopyranosyl.
Indicate the Carbons in Bond:
In parenthesis, indicate the carbons involved in the bond: (1→4).
Name the Second Residue:
The second glucose unit, which is a standard D-glucopyranose, is simply referred to as D-glucopyranose.
Complete Name
Putting this all together for maltose, we get:
α-D-glucopyranosyl-(1→4)-D-glucopyranose
**furano or pyrano for carbon ring
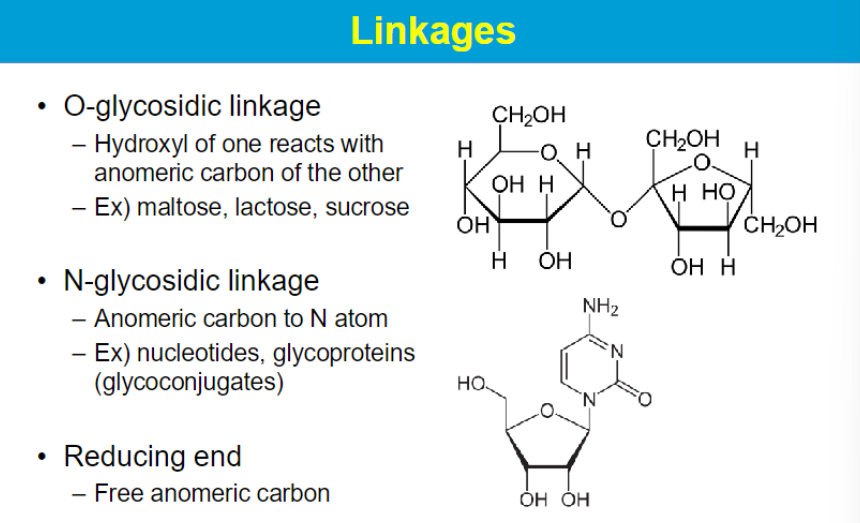
other disaccharide examples
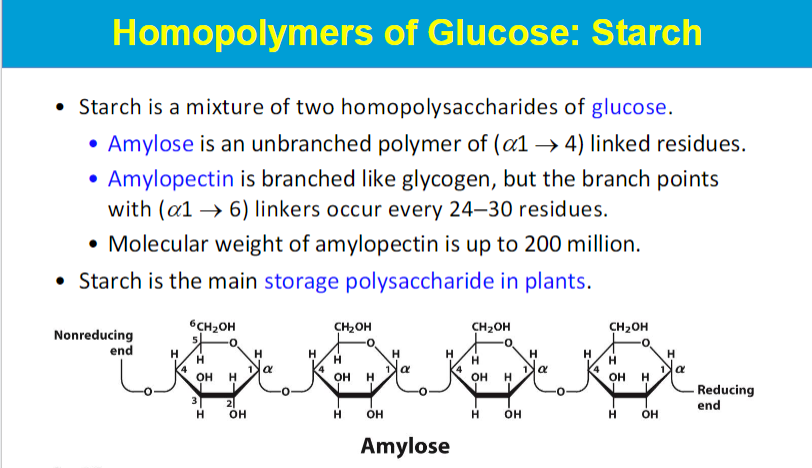
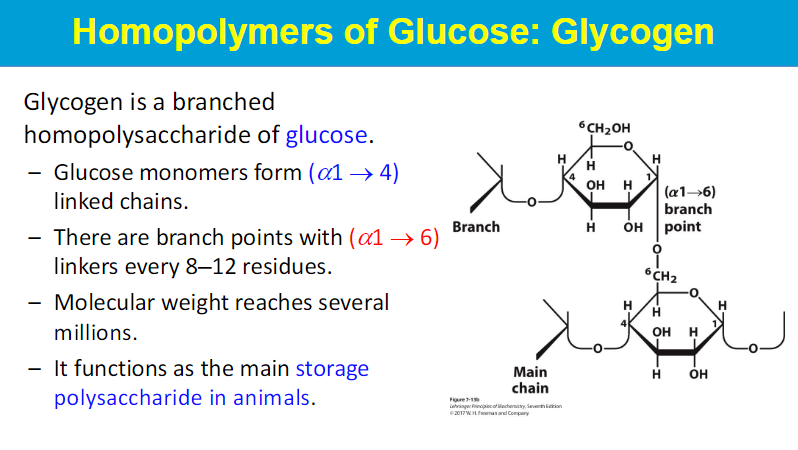
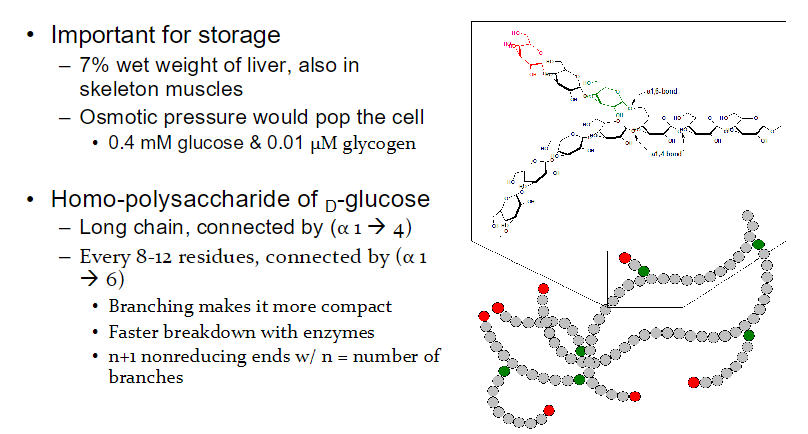
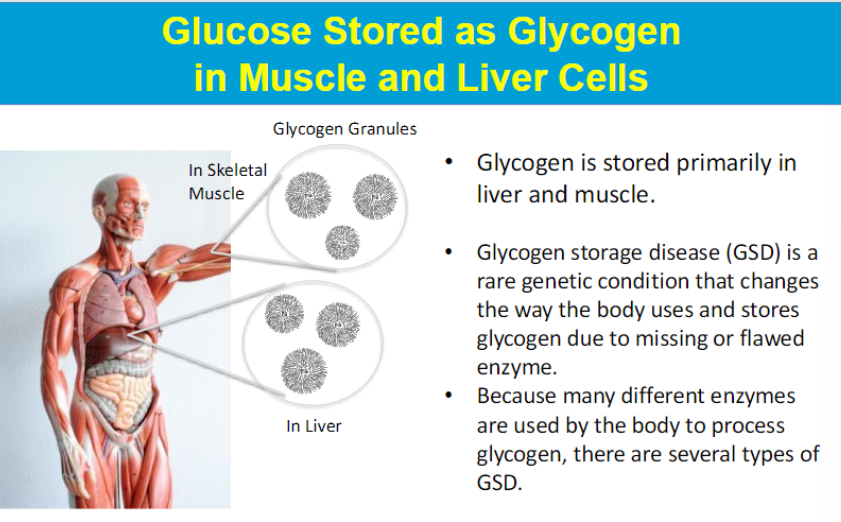
highly branched
synthesized and degraded for energy as demanded
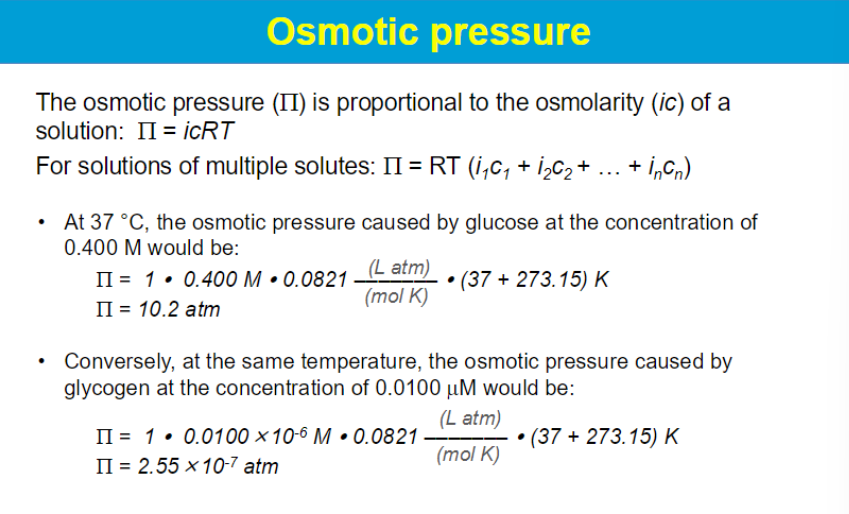
i: is the van't Hoff factor C: is the molar concentration of the solute
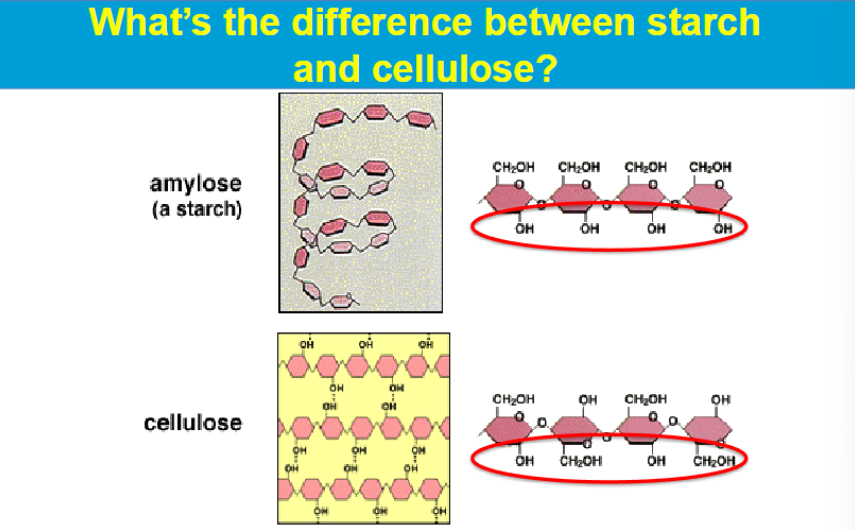
BETA
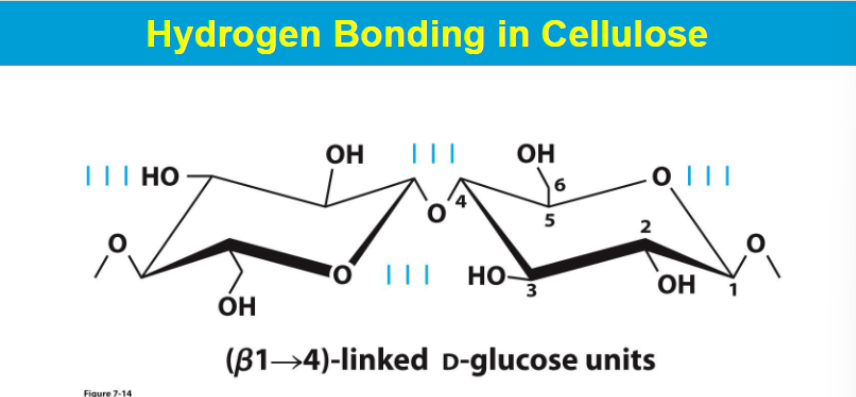
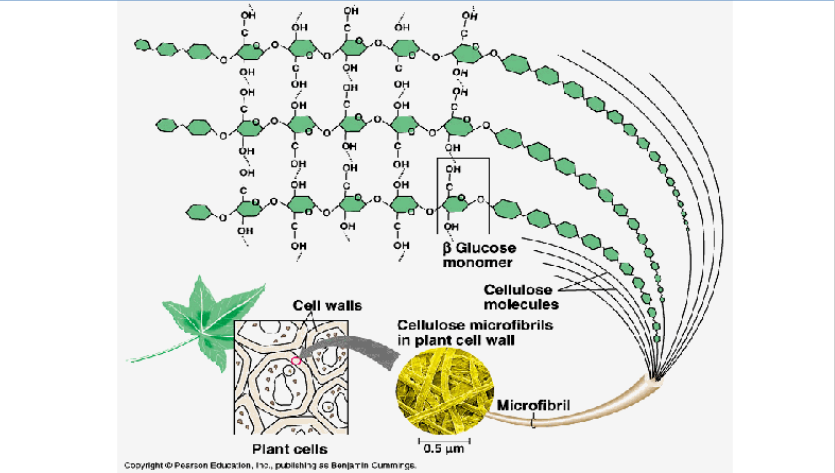
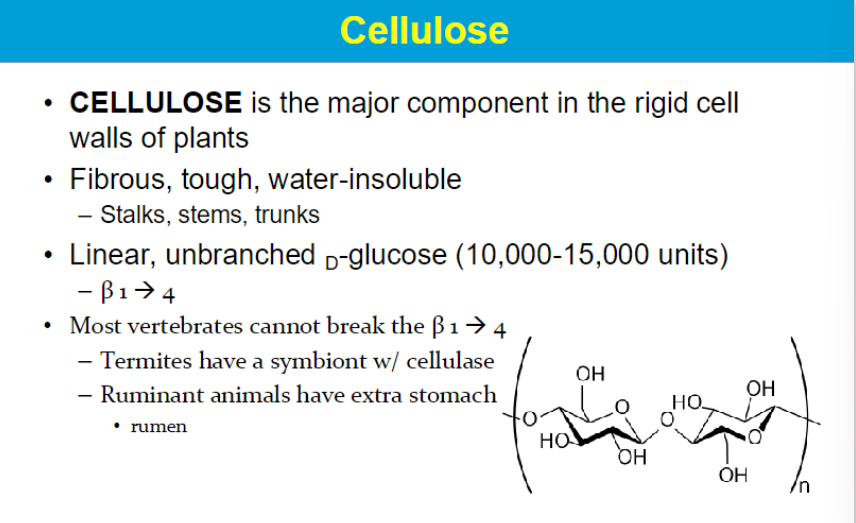
CELLULOSE IS A Structural Polysaccharides
CELLULOSE is the most abundant organic compound on earth
Cellulose can’t be digested by most animals so it passes through our digestive tract undigested. What’s it called?
FIBER
We lack the correct enzyme in our intestines…
Therefore, cellulose is NOT considered a nutrient.