Action Potential
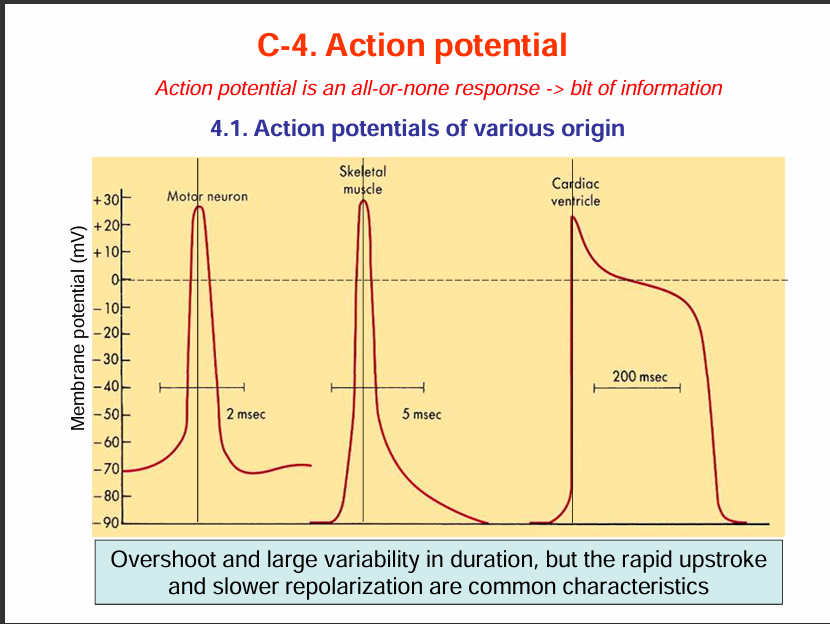
Action potential is an all or none response.
Action potentials have different shapes depending on the type of tissue.
The action potential graph for motor neuron and skeletal muscles look similar but the difference is that for skeletal muscle, it lasts longer (5msec) while for motor neuron it is 2msec.
Also the resting membrane potential for motor neuron is -70mV while that of skeletal muscle is -90mV.
For cardiac muscles, the duration of the action potential us 200msec.
Some similiaries and Differences:
Similarities: Rapid upstroke and slower repolarisation.
Differences: Differences in their duration of action potential and their overshoot.
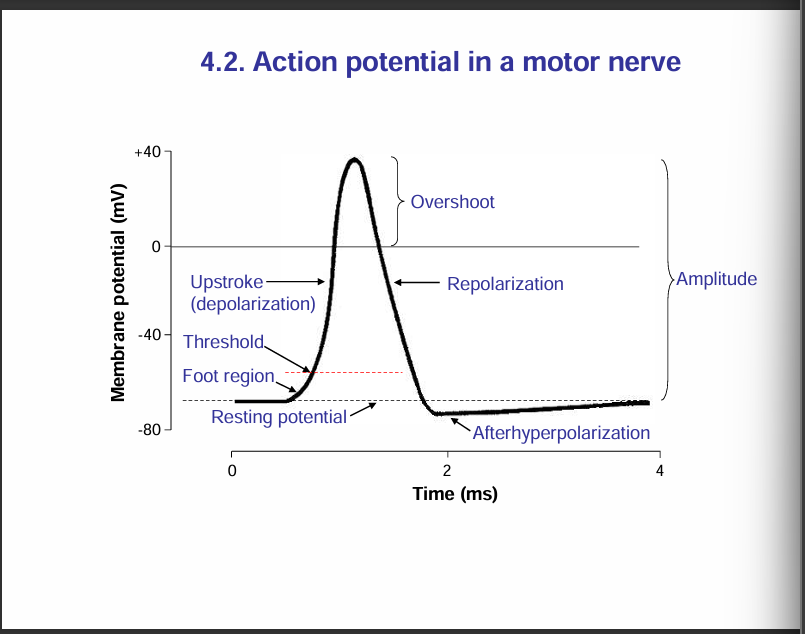
Action potential in a motor nerve:
Nerve signals are transmitted by action potentials, which are rapid changes in the membrnae potential that spread rapidly alon the nerve fibre membrane.
Each action potential begins with a sudden change from the normal resting membrane potential to a positive potential and then ends with an almost equally rapid change back to the negative potential.
To conduct a nerve signal, teh action potential moves along th nerve fibre until it comes to the fibres’s end
Note: "When the membrane potential reaches the threshold, an action potential is triggered."
Phases of an action potential in a motor nerve:
Resting stage: This is the resting membrane potential before the action potential begins. the membrane is said to be polarised during this stage.
Depolarisation stage: At this time, the membrane suddenly becomes permeable to sodium ions, allowing tremendous numbers of postively charged sodium ions to diffuse to the interior of the axon. The normal polarised state of -70mV is immediately neutralised by the inflowing positively charged sodium ions, with the potential rising rapidly in the positive direction. This is called depolarisation. In large nerve fibres, the great excess of Na+ ions moving to the inside causes the membrane potential to actually overshoot beyond the zero level and become somewhat positive. In some smaller fibres, as well as in many central nervous system neurons, the potential merely approaches the zero level and does mot overshoot to the postive state.
Repolarisation stage: In this stage, the sodium ion channels begin to close and the potassium ion channels open more than normal. Then rapid diffusion of potassium ions to the exterior re-establishes the normal negative resting membrane potential. This is called repolarisation of the membrane.
After hyperpolarisation:
During an action potential, the neuron first depolarises (becomes more positive), then repolarises (returns toward resting potential).
But instead of stopping exactly at the resting potential, the membrane potential briefly becomes more negative than the resting level.
This happens because voltage-gated potassium (K⁺) channels stay open a little longer than needed, allowing extra K⁺ ions to leave the cell.
Eventually, these channels close and the resting potential is restored.
Take note:
Amplitude of an action potential is measured as the difference between the resting membrane potential (usually around –70 mV) and the peak of the depolarisation/overshoot.
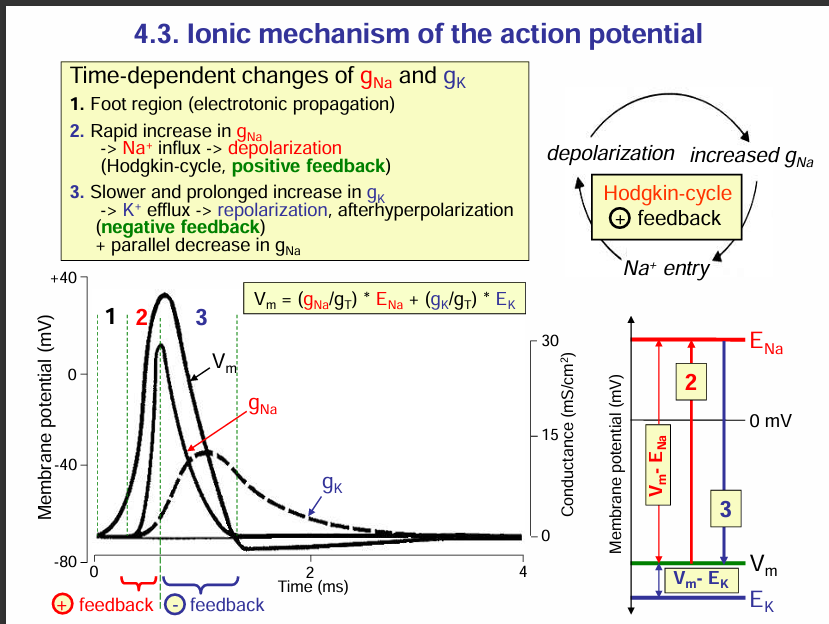
Ionic mechanism of the action potential
Time dependent changes in the conductance of Na+ and K+ ions:
Foot region (electrotonic propagation): The foot region is the region due to electrotonic potentials. This region is the region before the threshold potential.
Rapid increase in the conductance of Na+: Na+ ion channels open and thus Na+ ions enter into the axon. this causes depolarisation of the axon. More sodium ion channels open this more sodium ions enter into the axon of the neuron. This is a positive feedback (Hodgkin cycle). SEE EXPLANATION BELOW.
Note: At the peak of the depolarisation i.e. the overshoot point, the Na+ ions beome inactive. This imactivation prevents further Na+ entry, and at the same time, the voltage-gated K+ channels open, allowing K+ to exit the cell.
Slower and prolonged increase in K+ ion conductance: the voltage-gated K+ channels open thus leading to efflux of K+ ions. This causes repolarisation then after hyperpolarisation (negative feedback) then the cell returns to its resting membrane potential.
What is Hodgkin cycle:
This is a positive feedback cycle.
Here’s how it works step by step:
Depolarisation begins → A stimulus causes a small depolarisation (electrotonic potential).
Voltage-gated Na⁺ channels open → This allows Na⁺ to rush into the cell.
More depolarisation occurs → The influx of Na⁺ makes the inside even more positive.
Positive feedback loop → This additional depolarisation opens more Na⁺ channels, causing even more Na⁺ influx.
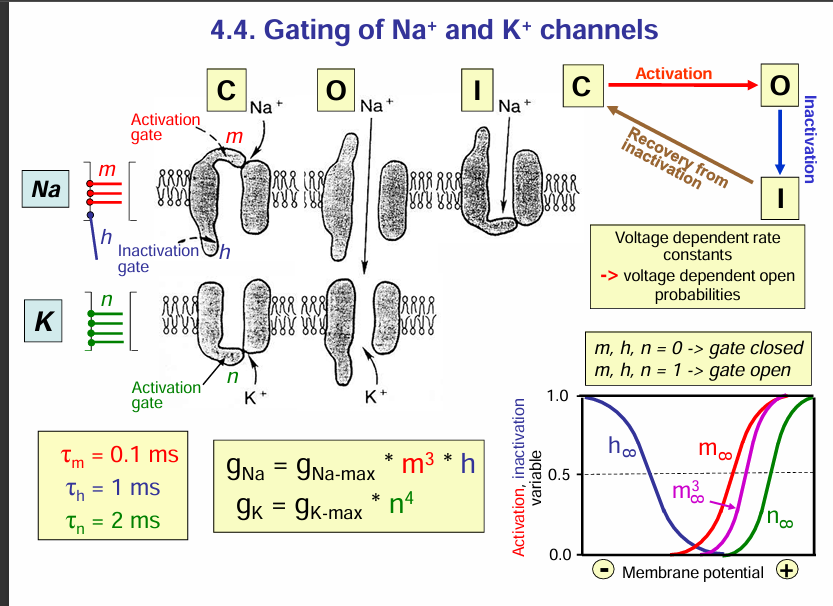
Gating of Na+ and K+ channels:
Na+ and K+ channel:
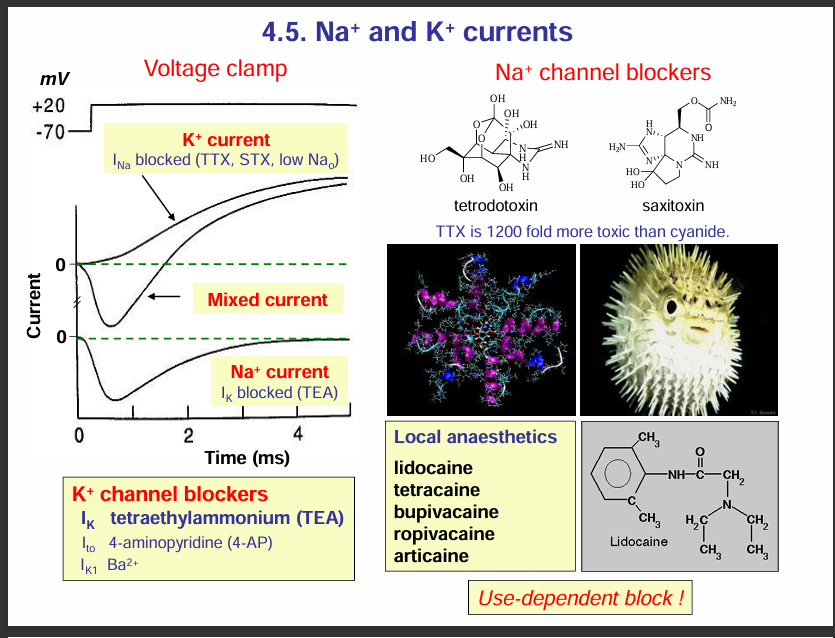
Na+ channel blockers:
If Na+ channels are blocked, this would block depolarisation thus, no action potential occurs.
Example of these blockers include: tetrodoxtoxin and saxitoxin. These toxins can cause suffocation
HOW THIS CAN CAUSE SUFFOCATION.
If Na⁺ channels are blocked, the Hodgkin cycle cannot occur, so no action potential can be generated or propagated.
In the case of the phrenic nerve (which controls the diaphragm), failure to generate action potentials means the diaphragm doesn’t contract.
Without diaphragm contraction, breathing stops → leading to respiratory paralysis and suffocation.
Local anaesthetics:
They are used to numb the pain during minor surgeries or dental procedures . These anasthetics work by blocking voltage-gated Na+ ion channels in sensory nerves. This prevents depolarisation and action potential generation so pain signals cannot be transmitted to the central nervous system.
Examples of local anaesthetics include:
Lidocaine
Tetracaine
Bupivacaine
Ropivacaine
Articaine
K+ channel blockers:
They block/ prevent repolarisation
Tetraethylammonium (TEA)
4-aminopyridine (4-AP)
Ba2+
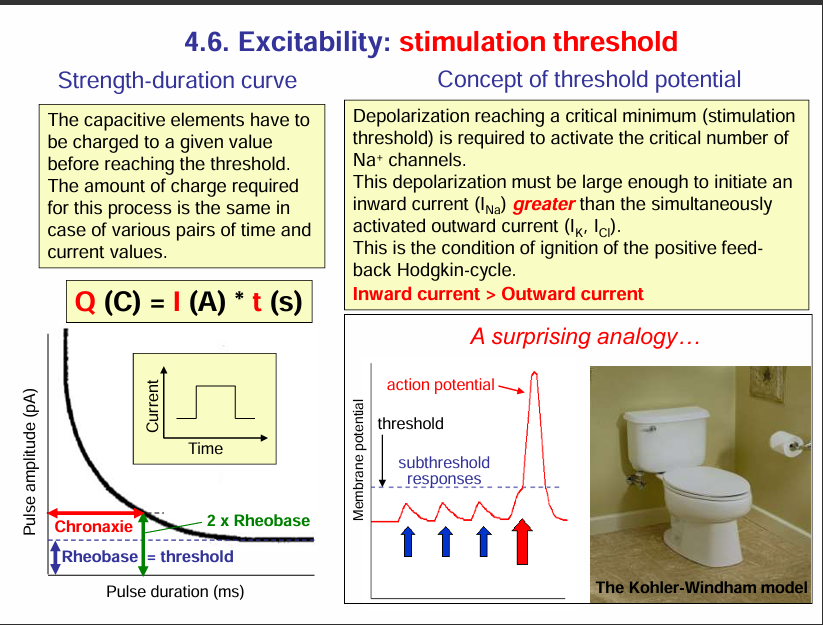
Excitability: stimulation threshold
This slide is about excitability and the stimulation threshold — basically, what’s needed to trigger an action potential. Let’s break it down section by section:
1. Strength–Duration Curve (bottom left)
To excite a neuron/muscle, you need to deliver a certain charge (Q).
That charge can come from different combinations of current (I) and time (t) → Q=I×tQ = I \times t.
The curve shows that:
A short pulse requires a large current.
A longer pulse requires only a smaller current.
Two key terms here:
Rheobase = the minimum current needed to trigger an action potential if applied for a very long duration. It is the lowest possible current that can generate an action potential. The unit of rheobase is picoamperes.
Chronaxie = the time required to reach threshold if current is twice the rheobase. So it is the time for 2 times the rheobase. The unit for Chronaxie is milliseconds.
These values describe how excitable a nerve/muscle is.
2. Threshold Potential (top right)
To fire an action potential, the inward sodium current (I_Na) must become greater than the outward currents (mainly K⁺, Cl⁻).
Once this balance tips, the Hodgkin cycle (positive feedback loop) kicks in:
depolarisation → more Na⁺ channels open → more depolarisation → action potential.In short:
Subthreshold stimulus = depolarisation is too small, no action potential.
Threshold stimulus = depolarisation just enough to start the Hodgkin cycle.
Suprathreshold stimulus = bigger depolarisation, but action potential size is always the same (all-or-none).
3. Toilet Analogy (bottom right)
The toilet illustrates the all-or-none law:
If you don’t press hard enough on the flush (subthreshold), nothing happens.
Once you reach the threshold (enough push), the flush completes fully (like an action potential firing).
Pushing harder doesn’t make the flush bigger → just like an action potential, which is always the same size once triggered.
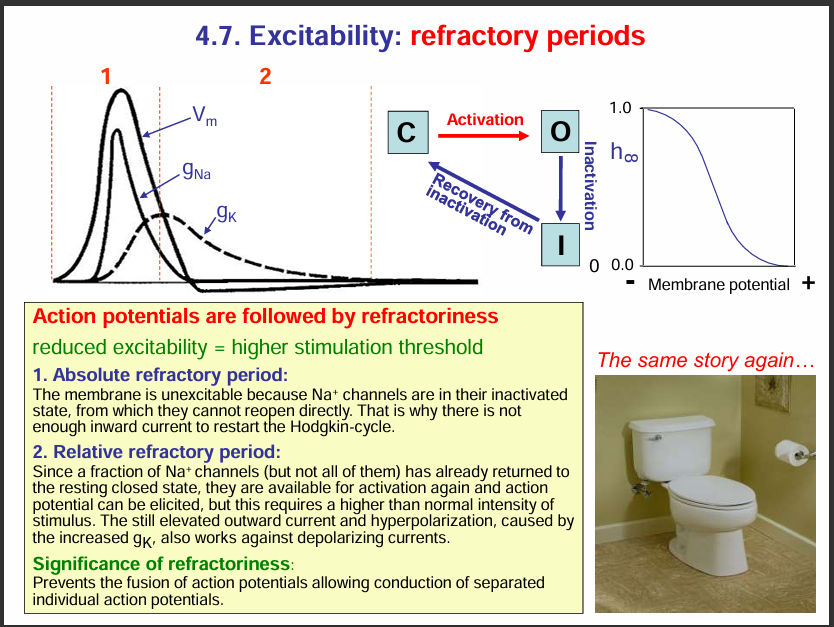
Refactory period:
During the refactory period, the axon is less excitable
Types:
Absolute refactory period: The sodium channels are inactivated so they cannot be stimulated to open since they are inactive.
Relative refactory period: A fraction of the Na+ ion channels are closed so they can get stimulated and can open to allow Na+ ion influx. Also K+ ion channels are also open and thus for an action potential to be generated, a very strong stimuli is needed to overcome the repolarisation by the K+ ion channels.
WHY IS THE REFRACTORY PERIOD IMPORTANT:
It prevents the fusion of action potential allowing the conduction of separate individual action potentials.
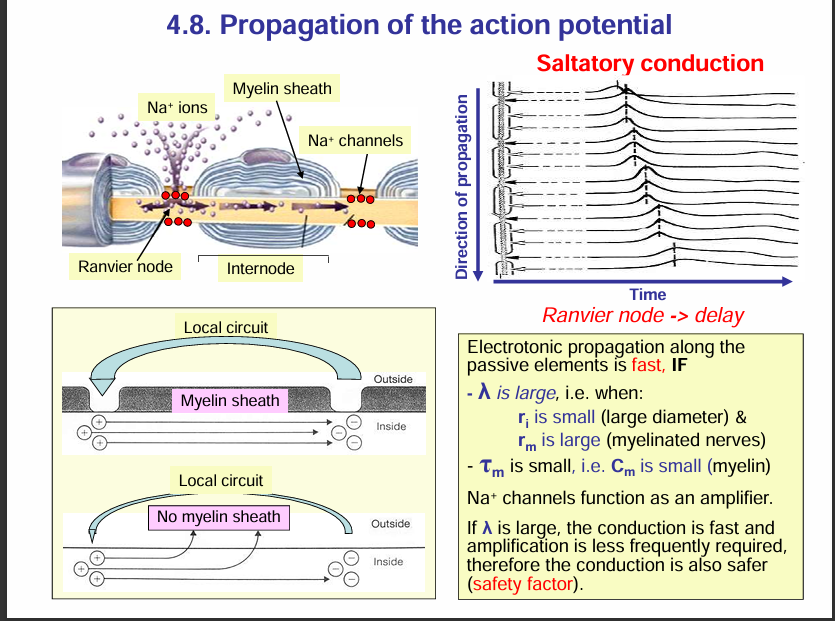
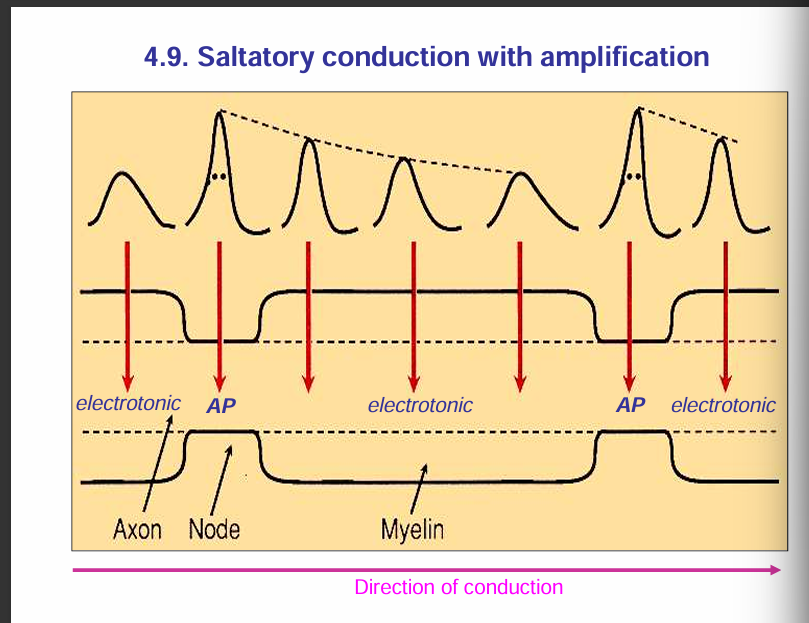
Propagation of an action potential:
🔹 Nodes of Ranvier (Active Membrane)
Rich in voltage-gated Na⁺ channels.
When depolarisation reaches a node, these Na⁺ channels open → Na⁺ rushes in → action potential is actively generated.
Because of this, each node acts like a booster station for the signal.
🔹 Internodal Regions (Myelinated Segments = Passive Membrane)
Covered by myelin sheath → prevents ion exchange across the membrane.
These regions have very few or no voltage-gated Na⁺ channels.
Instead of regenerating an action potential, the depolarisation here just spreads passively (electrotonically) through the axoplasm under the myelin.
Passive spread would normally decay, but since the next node is close, the depolarisation is still strong enough to reach threshold and regenerate the action potential there.
🔹 Overall Process = Saltatory Conduction
Action potentials are actively regenerated at the nodes.
They passively “leap” through the myelinated internodes.
This combination makes conduction both fast and energy-efficient.
✅ So your statement is correct:
Nodes = active (AP generated).
Internodes = passive (electrotonic conduction).
Take note: To increase conduction, large diameter (small ri) and myelination (large rm) is needed.
rm= membrane restistance
ri = internal resistance of axon.
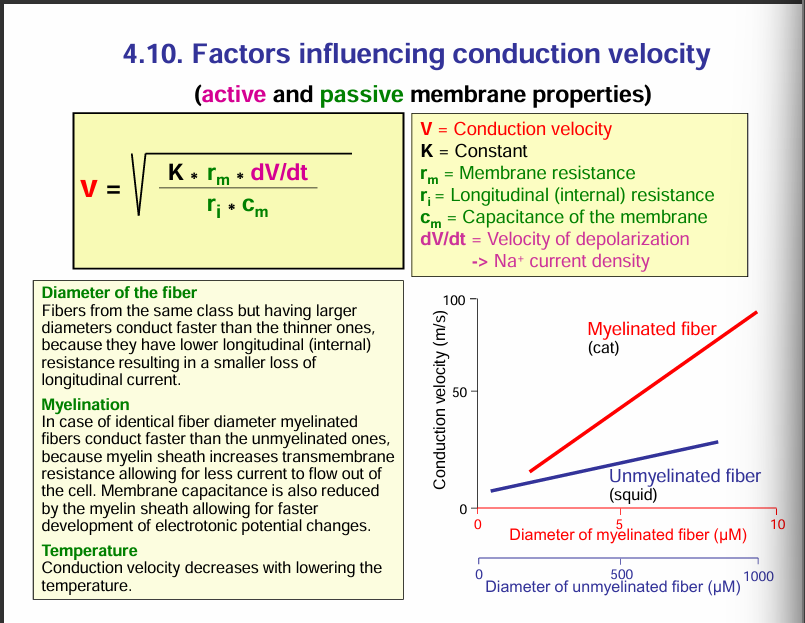
Factors influencing conduction velocity:
rm = membrane restistance. This can be increased by myelination. Increasing the membrane resistance would increase the velocity of the conduction. Myelination increases the transmembrane resistance thus allowing less current to flow out of the axon.
ri = internal restistance. Decreasing the internal resistance would increase the conduction velocity. This can be done by increasing the diameter of the axon.
Cm= reducing membrane capacitance increases the conduction velocity. The membrane capacitance is also reduced by the myelin sheath allowing for faster development of electrotonic potential changes. MYELINATION INCREASES MEMBRANE RESISTANCE AND DECREASES CAPACITANCE.
Temperature: conduction velocity decreases with lowering the temperature.
NOTE:
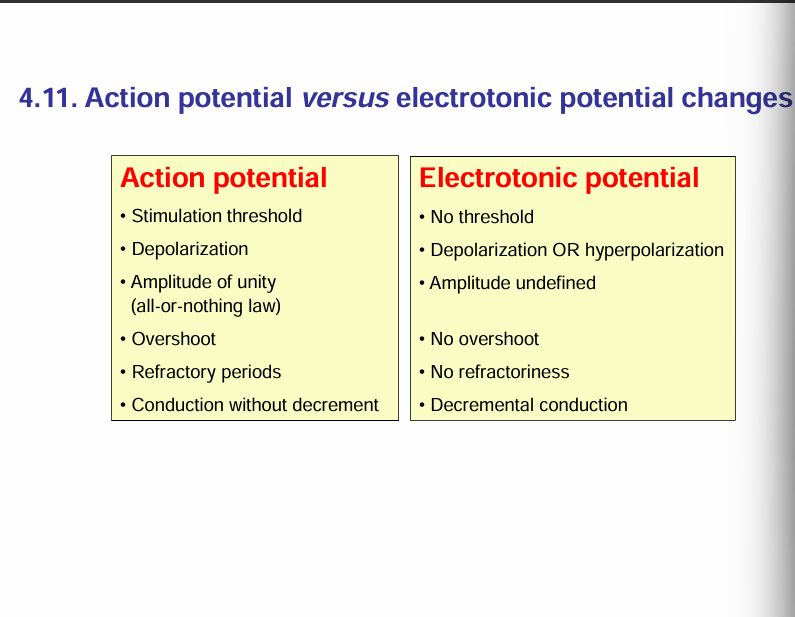
Action potentials require an active membrane to be generated/ An active membrane is a membrane that has voltage-gated Na+ channels.
For electrotonic potential, a passive membrane is required. A passive membrane does not have volatage-gated Na+ channels.