6.5-6.7 Membrane Potentials
6.5 Basic Principles of Electricity in Physiology
Physiological processes follow chemistry and physics laws.
Net flux of charged molecules is key.
Extracellular fluid: predominantly high in sodium (Na^+) and chloride (Cl^-) ions.
Intracellular fluid: High in potassium (K^+) ions and nonpenetrating ionized molecules (e.g., phosphate compounds, negatively charged proteins).
Electrical phenomena at plasma membrane are vital for signal integration and cell communication in neurons.
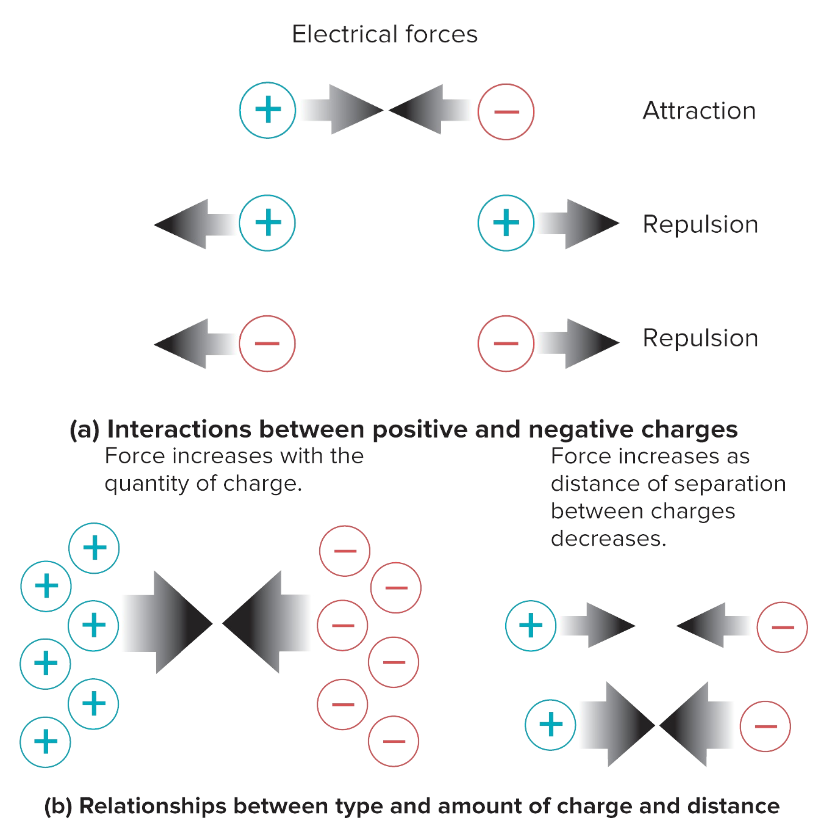
Like charges repel; opposite charges attract.
Electrical Potential and Current
Separated opposite charges have electrical potential.
Electrical potential: Potential to do work if charges come together; also known as potential difference.
Unit: Volts (V).
Biological systems: Use millivolts (mV), where 1 \, mV = 0.001 \, V .
Current: Movement of electrical charge, driven by electrical potential.
opposite charges → current brings both charges together
alike charges → current separates them
Current magnitude:
amount of charge that moves
depends on the potential difference and the material through which charges move.
Resistance: Hindrance to charge movement; high resistance = low current.
Ohm’s Law: I = \frac{V}{R} (Voltage (V), current (I), and resistance (R)).
High electrical resistance (insulators) reduce current flow. Lipids act as insulators in cell membranes.
Low resistance (conductors) allow rapid current flow. Intracellular and extracellular fluids with ions are conductors.
Lipid layers of plasma membrane provide high electrical resistance.
6.6 The Resting Membrane Potential
Neurons at rest: Have a potential difference across plasma membrane (V_m).
known as the resting membrane potential
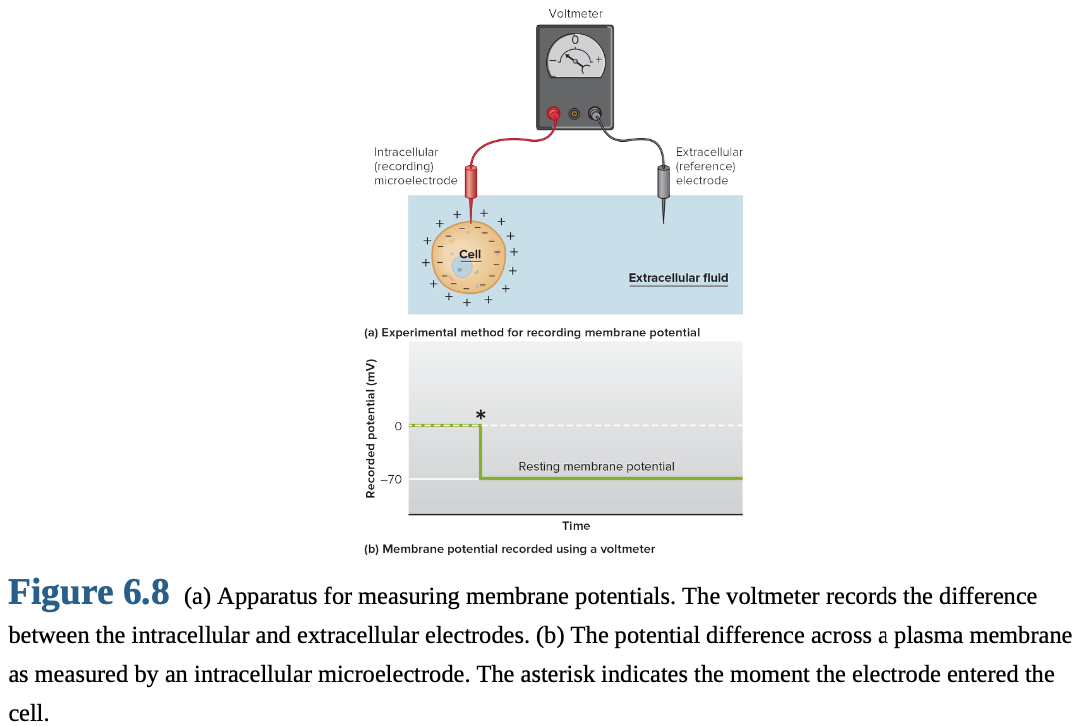
Inside of cell: Negatively charged relative to outside.
Extracellular fluid: Voltage reference point; membrane potential polarity is relative to it.
Example: -70 mV means inside is 70 mV more negative than outside.
V_m magnitude: Typically -40 to -90 mV in neurons.
Nature and Magnitude of Resting Membrane Potential
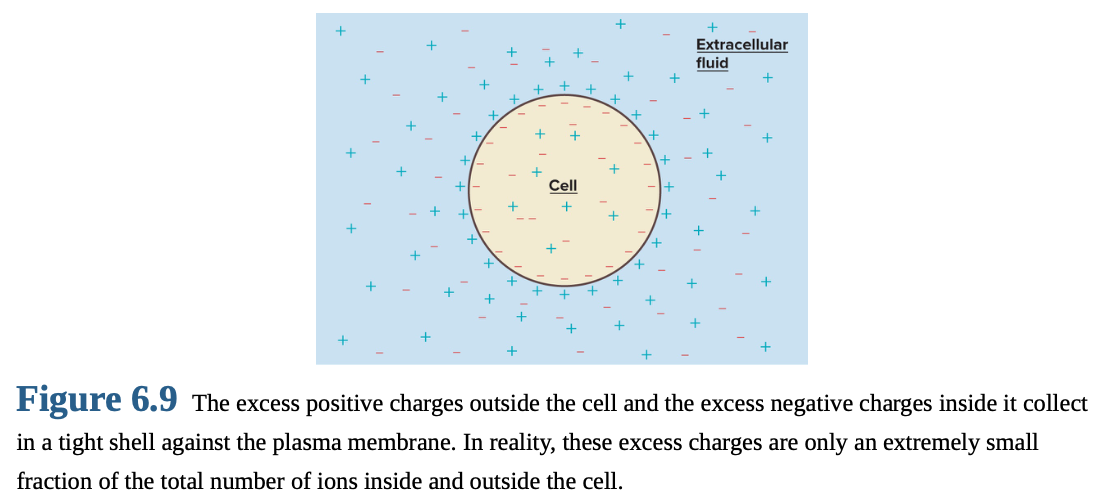
Resting membrane potential exists due to excesses of negative ions inside the cell and positive ions outside.
Excess charges accumulate in thin shell along plasma membrane surfaces.
Number of charges needed to establish potential is a tiny fraction of total charges.
Ion Distribution
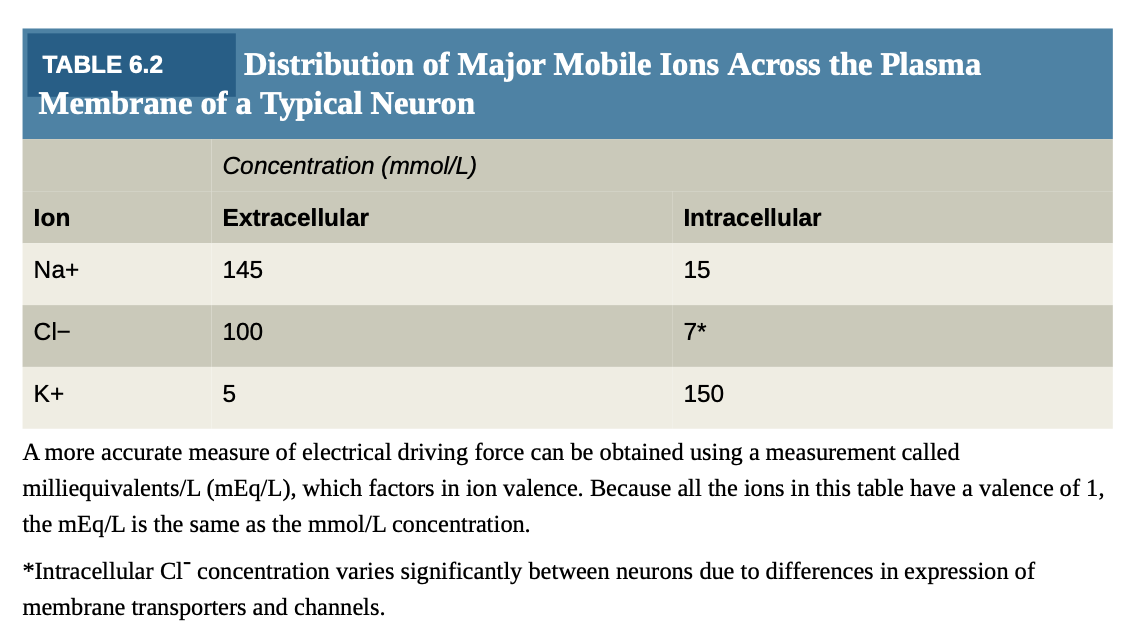
Sodium (Na^+) and chloride (Cl^-) concentrations lower inside than outside; potassium (K^+) higher inside.
Sodium/potassium-ATPase pump (Na^+/K^+-ATPase ): Maintains gradients by pumping Na^+ out and K^+ in.
Chloride distribution varies among cell types.
Electrical driving force measured using milliequivalents/L (mEq/L).
Factors Affecting Magnitude of Resting Membrane Potential
Differences in ion concentrations in intracellular and extracellular fluids.
Differences in membrane permeabilities to ions via open channels.
Direct role of ion pumps plays a minor role.
Contribution of Ion Concentration Differences
Membrane permeable only to K^+ (with K^+ channels open).
Initially, compartments have equal ion concentrations, no potential difference.
K^+ diffuses down gradient (high to low), creating potential difference.
Compartment 1 becomes positively charged, compartment 2 negatively charged.
Membrane potential influences K^+ movement; negative charge attracts K^+.
Electrochemical gradient exists for all ions.
Equilibrium potential for K^+ when fluxes balance; no net movement.
Membrane potential generated with concentration gradient and open K^+ channels.
Equilibrium established by insignificant ion number.
Equilibrium potential magnitude depends on ion concentration gradient.
Nernst Equation
Calculates equilibrium potential using ion concentration gradient.
E{ion} = 61 \times log\left(\frac{C{out}}{C_{in}}\right)
E_{ion} = equilibrium potential for a particular ion (mV)
C_{in} = intracellular concentration of the ion
C_{out} = extracellular concentration of the ion
z = valence of the ion
61 = a constant value incorporating the universal gas constant, temperature (37°C), and Faraday electrical constant
Typical concentrations: Na^+ flux brings membrane potential to +60 mV, K^+ flux to -90 mV.
Contribution of Different Ion Permeabilities
Membrane potential depends on permeabilities and concentration gradients of all ions when multiple channels are open.
Greater the membrane permeability to an ion, the greater its contribution to the membrane potential.
Goldman-Hodgkin-Katz (GHK) equation calculates resting membrane potential (Vm): Vm = 61 \times log\left(\frac{PK[K^+]{out} + P{Na}[Na^+]{out} + P{Cl}[Cl^-]{in}}{PK[K^+]{in} + P{Na}[Na^+]{in} + P{Cl}[Cl^-]{out}}\right)
GHK equation accounts for individual ion permeabilities.
Ion gradients and permeabilities vary in different excitable cells.
Mammalian neurons: K^+ permeability is much greater than that for Na^+ and Cl^- , so the resting membrane potential is close to the equilibrium potential for K^+.
Chloride (Cl^-) has minimal importance compared to K^+ and Na^+.
Resting Potential Generation
Largely by K^+ movement out of cell via leak channels, making inside negative.
Resting membrane potential not equal to K^+ equilibrium potential because some open leak channels for Na^+ pull the membrane potential towards the Na^+ equilibrium potential.
The Na^+/K^+-ATPase pump maintains stable concentrations of intracellular sodium and potassium ions by balancing the leak of ions down their electrochemical gradients.
Contribution of Ion Pumps
Na^+/K^+-ATPase pump maintains concentration gradients.
Pump directly contributes to negative resting potential by moving three Na^+ out for every two K^+ in (electrogenic pump).
Electrogenic contribution is small but vital.
Development of Resting Membrane Potential
Na^+/K^+-ATPase pump establishes concentration gradients, determining equilibrium potentials, and has small electrogenic effect.
Greater K^+ efflux than Na^+ influx results in negative membrane potential due to higher permeability to K^+.
Steady-state: Dynamic balance is reached with equal inward and outward currents maintaining a steady membrane potential.
The Na^+/K^+-ATPase pump balances ion movement to maintain concentration gradients.
In cells without chloride pumps, Cl^- concentrations shift until the equilibrium potential for Cl^- equals the resting membrane potential.
Some cells have nonelectrogenic active-transport systems that move Cl^- out of the cell, creating a Cl^- equilibrium potential negative to the resting membrane potential, contributing to the excess negative charge inside the cell.
Key Concepts
Resting membrane potential: Electrical potential difference across plasma membrane, generated by ion concentration differences and membrane permeabilities.
Equilibrium potential: Membrane potential at which concentration and electrical forces on an ion are equal.
GHK equation: Calculates membrane potential with known ion concentrations and permeabilities.
Na+/K+-ATPase pumps: Maintain low intracellular Na^+ and high intracellular K^+ concentration via active transport; contribute directly as an electrogenic pump.
Graded Potentials and Action Potentials
Cells generate electrical signals via gated ion channels, changing membrane potential.
Excitability: Ability of a cell to produce electrical signals.
Graded potentials: Important for short-distance signaling.
Action potentials: Long-distance signals in neuronal and muscle cell membranes.
Terms Describing Changes in Membrane Potential
Depolarize: Membrane potential becomes less negative.
Overshoot: Reversal of membrane potential polarity.
Repolarize: Membrane potential returns to resting value.
Hyperpolarize: Membrane potential becomes more negative.
Graded Potentials
Changes in membrane potential confined to small region.
Magnitude varies.
Examples: receptor, synaptic, and pacemaker potentials.
Local current flow spreads depolarization.
Charge is lost across the membrane, and potential decreases with distance.
Local current flow is decremental, decreasing with distance.
Summation: Additional stimuli add to potential.
Action Potentials
Significant changes in membrane potential.
Rapid and repeat at high frequencies.
Mechanism: Propagation down the axon is communication in the nervous system.
Voltage-Gated Ion Channels
Regulate ion channel opening.
Mediate graded potentials as initial stimulus for action potential.
Types: Conduct Na^+, K^+, Ca^{2+}, Cl^-; behavior varies with voltage.
Depolarization opens channels; negative potential keeps them closed.
Sodium channels open faster than potassium channels.
Voltage-gated Na+ channels contain an inactivation gate, which limits Na+ flux by blocking the channel shortly after depolarization opens it.
Action Potential Mechanism
Transient changes in membrane permeability allow ions to move down gradients.
Depolarizing stimulus: Neurotransmitter binds to ligand-gated ion channel, allowing Na^+ entry.
Positive Feedback Loop: Na^+ entry causes depolarization, opening more voltage-gated Na^+ channels.
Absolute Refractory Period: Na^+ permeability declines as inactivation gates block open Na^+ channels.
Voltage gated K^+ channels open: Increases K^+ flux out of the cell - repolarizes the membrane toward its resting value
Afterhyperpolarization – K^+ permeability remains above resting levels and the membrane is transiently hyperpolarized toward the K^+ equilibrium potential
Voltage-gated Na+ channels operate in a positive feedback mode at the action potential's beginning, while voltage-gated K+ channels trigger the action potential's conclusion via a negative feedback process.
Threshold Potential
Action potentials occur only when stimulus elevates membrane potential beyond threshold.
Threshold stimuli are strong enough to depolarize the membrane to the threshold potential.
Subthreshold potentials: Weak depolarizations, no action potential.
Stronger stimuli elicit the same amplitude action potentials.
All-or-None Principle
Action potentials occur maximally or not at all.
Frequency and patterns of action potentials provide stimulus magnitude information.
Local anesthetics (e.g., procaine, lidocaine) block voltage-gated Na^+ channels, preventing transmission of graded signals.
Refractory Periods
Absolute Refractory Period: Further stimulation won't cause another action potential because Na^+ channels are open or inactivated.
Relative Refractory Period: A second action potential can be elicited only if the stimulus strength is greater than normal due to some Na^+ channels being in a resting state and K^+ channels still being open.
Refractory periods limit the number of action potentials an excitable membrane can produce in a given time.
Action Potential Propagation
Action potential travels the neuron if each point is depolarized to threshold.
Potential differences cause current flow, depolarizing adjacent membrane and opening voltage-gated Na^+ channels.
Each regeneration depends on the positive feedback cycle of a new group of Na^+ channels.
Action potentials are not decremental
Action potential propagation is away from a region of membrane recently active.
Excitatory membranes can conduct action potentials in either direction if the membrane through which the action potential must travel is not refractory
Myelination
Action Potential Velocity: Depends upon fiber diameter and myelination.
Membrane pumps need to restore fewer ions; less charge leaks, and more arrives at the adjacent node.
Myelinated axons are metabolically more efficient.
Action potentials appear to jump from node to node - saltatory conduction.
Conduction velocities range from 0.5 m/sec for small-diameter, unmyelinated fibers to 100 m/sec for large-diameter, myelinated fibers.
Generation of Action Potentials
Threshold potential must be attained.
In afferent neurons, receptor potentials generate initial depolarization
Action Potential Initiators:
Synaptic input to the neuron, known as a synaptic potential.
Spontaneous change in the neuron’s membrane potential, known as a pacemaker potential.
Key concepts
*Neurons are excitable
*Graded potentials - local potentials with magnitudes that can vary are decremental
*Action potential - large change in membrane potential- membrane depolarizes and then repolarizes
*Myelination results in Saltatory conduction – regeneration of action potentials only at nodes of Ranvier