Chapter 4 - Chemical Bonding
4.1 - Chemical Bonds and Greenhouse Gases
- Average temperature of the Earth’s surface is increasing and this increase is directly correlated to the increasing atmospheric concentrations of greenhouse gases such as CO2 and CH4. Carbon dioxide is especially good at trapping heat because it absorbs infrared radiation because of the unequal sharing of electrons within its bonds.
Ionic Bonds: cation from a metallic element and an anion from a nonmetal
Couloumbic attraction: electrostatic attractions between ions of opposite charge
- The value of electrostatic potential energy is inversely related to the distance; the greater the attraction (higher charge or shorter distance between ions) the more negative the electrostatic potential energy value
Lattice Energy (U): energy released when free, gas-phase ions combine to form one mole of a crystalline solid
Crystal Lattice: ordered 3-D array of particles
Covalent Bonds: equal “sharing” electrons
- characterized by a drop in potential energy at the distance where attraction strength between one nucleus and each of the electrons equal the strength of attraction between second nucleus and its electrons
Bond length: distance between two nuclei in the bond
Bond energy: amount of energy required to break one mole of bond into two moles of the free atom
Metallic Bonds: overlapping valence-shell orbitals characterized by the nucleus-surrounding electron attraction
- “sea” of mobile electrons that flow freely between all atoms in a piece of metal
- allows for conduction of electricity
4.2 Electronegativity, Unequal Sharing and Polar Bonds
Polar Covalent Bond: unequal sharing of electrons between atoms; 2.0>Δχ>0.4
- Dipole: a pair of opposite charges separated by a distance; arrow points towards the more negative, electron-rich end of the bond
- δ +/- represent the partial electrical charges
- total electrical charge: complete transfer of electrons; Δχ>2.0
Nonpolar Covalent Bond: even distribution of charge, equal sharing of electrons; Δχ>0.4
Electronegativity: an atom’s tendency to attract electrons toward itself in a chemical bond
4.3 Naming Compounds and Writing Formulas
Binary Molecular Compounds: two nonmetals
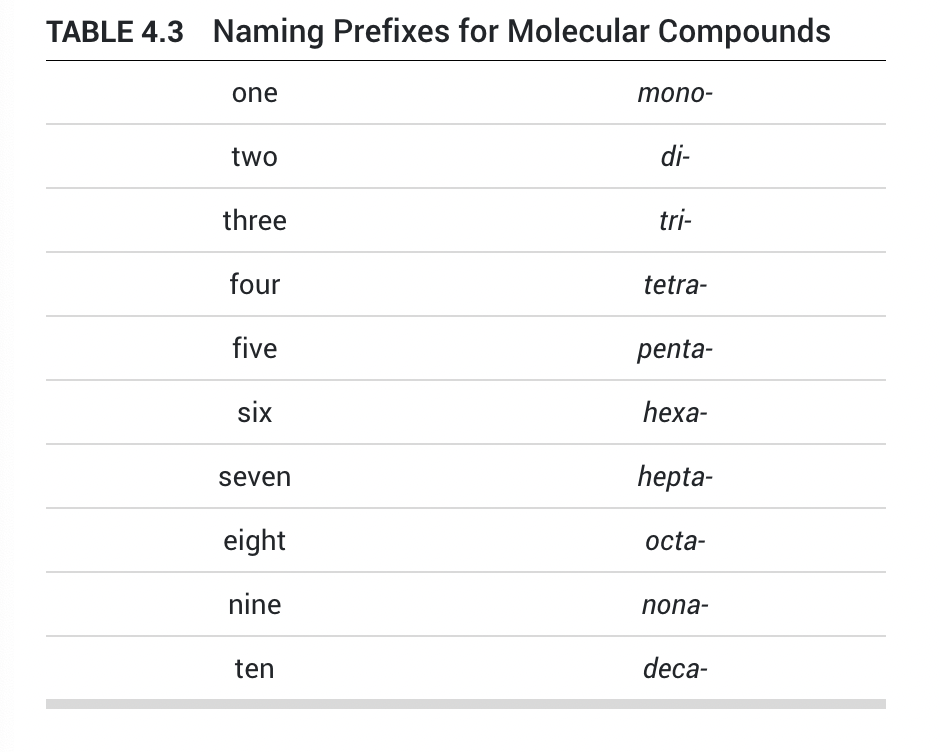
name first element in formula with prefix (emitting mono-)
name second element in formula with prefix, changing the ending to -ide
ex. SO2 is sulfur dioxide
Binary Ionic Compounds of Main Group Elements
- cation: name of parent element
- anion: name of parent element ending in -ide
ex. NaCl is sodium chloride
Binary Ionic Compounds of Transition Metals
same rules as the previous ionic compounds but the charge is specified with a roman numeral
ex. CuO is Copper(II) oxide
Binary Acids
add prefix hydro- to the name of the second element in the formula
replace last syllable in second element’s name with suffix -ic and add “acid”
ex. HCl (aq) is hydrochloric acid
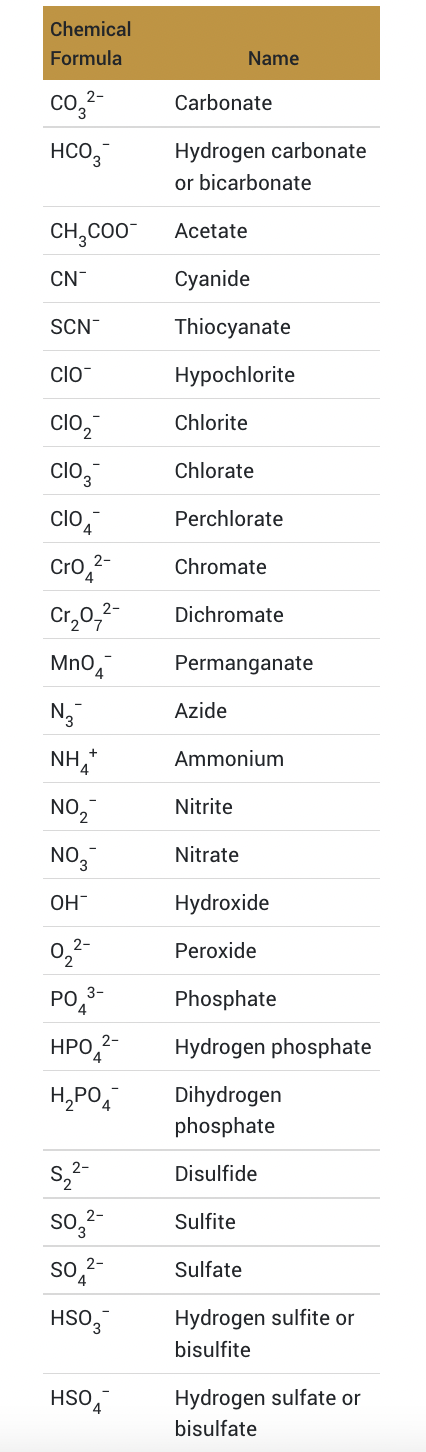
Polyatomic Ions
oxoanion with the most oxygen has prefix per- and suffix -ate; oxoanion with fewest oxygens has prefix hypo- and suffix -ite
ex. perchlorate (ClO4-), chlorate (ClO3-), chlorite (ClO2-) and hypochlorite (ClO-)
Oxoacids
-ate becomes -ic and -ite becomes -ous; add acid
ex. HNO2 is nitrous acid, HNO3 is nitric acid
 4.4 Lewis Symbols and Structures
4.4 Lewis Symbols and Structures
Octet rule: all atoms except the very smallest (ex. H) tend to lose, gain or share electrons to obtain 8 valence electrons
Lewis symbols: depict an atom’s bonding capacity - the number of bonds an atom typically forms to complete its octet. Consists of the element symbol surrounded by dots on all four sides representing the valence electrons; all four sides must have one dot before electrons can be paired
Lewis structure: two-dimensional representation showing how atoms connect
- determine total number of valence electrons
- construct skeletal structure with least electronegative element in the center, then connect with single bonds
- complete octets of all atoms except hydrogen by adding lone pares
- compare valence in structure to valence calculated; adjust by creating double/triple bonds or adding lone pairs to the central atom
- add net charge in brackets if applicable
Single bond: two atoms share one pair of electrons
Double bond: two atoms share two pairs of electrons
Triple bond: two atoms share three pairs of electrons
Lone Pairs: pair of electrons that is not shared
4.5 Resonance
Allotropes: different molecular forms of the same element, with different physical and chemical properties
Resonance Structures: 2+ Lewis structures with same atomic arrangement but different arrangements of bonding electrons and lone pairs
- Bond length is an “average” of the two structures
Delocalization: spreading out of electron density over multiple atoms, reducing the potential energy of electrons, therefore lowering the energy of the molecule (resonance stabilization)
4.6 Lengths and Strengths of Covalent Bonds
Bond Length: distance between the nuclei of two bonded atoms; depends on atomic identity and number of bonds formed
Bond Order: number of pairs of electrons atoms share
- as bond order increases, bond length decreases
Bond Energy: energy need to break one mole of bonds in the gas phase, always a positive quantity because breaking bonds requires energy; increases as bond order increases
4.7 Formal Charge
Formal Charge: an unreal charge that assigns electrons to atoms within the molecule
- FC= (number of valence electrons) - (number of electrons in lone pairs - number of bonds)
- Ideal formal charge is 0 on each element, but if impossible, most negative charge should be on the most electronegative element with formal charges as close to zero as possible
4.8 Exceptions to Octet Rule
Electron Deficient molecules: Be, B and Al
Free radicals: compounds containing unpaired valence electrons, tend to be very reactive to try to acquire or share an electron
Expanded Octet: nonmetals in third row and below, such as P, S, Xe, etc.
- typically bonded to strongly electronegative elements such as F, O, Cl
- reduces formal charge to be closer to 0
4.9 Vibrating Bonds and Greenhouse Effect
covalent bonds vibrate slightly, causing stretching and building that can cause the absorption of infrared radiation
- symmetric stretch: infrared inactive
- asymmetric stretch: infrared active
- bending mode: infrared active