Membrane
Overview of membrane structure
Membranes surround cells and organelles. These membranes act as barriers controlling the exchange of materials between the internal and external environment. The membranes are composed of:
lipids (see subtopic B1.1) like phospholipids, glycolipids and sterols
proteins (see section B1.2.11–12)
small amounts of carbohydrates in the form of glycolipids and glycoproteins (see section B2.1.9–10).
These lipids and some of the proteins that compose the membrane are amphipathic molecules with both hydrophobic and hydrophilic regions. You can read about these molecules in subtopics B1.1 and B1.2.
Lipid bilayer
Lipids are essential components of membranes, and include three major classes of lipids – phospholipids, glycolipids (see section B2.1.9–10) and sterols like cholesterol (section B2.1.11–13).
Membrane phospholipids (see section B1.1.12–13) are the most abundant and diverse lipids. Let us quickly review the structure of phospholipid molecules.
A phospholipid molecule comprises a backbone composed of mostly glycerol, a three-carbon alcohol. Attached to the backbone are:
a negatively charged phosphate molecule linked to molecules like choline or serine forming a polar, hydrophilic, ‘head’ group. The polar head easily forms hydrogen bonds with water.
two non-polar fatty acid chains forming the hydrophobic ‘tails’. The fatty acids could be saturated or unsaturated. Unsaturated fatty acids result in kinks in the tail.
Click on the hotspots in Interactive 1 to learn more about the features of a phospholipid molecule.
Interactive 1. A schematic representation of a phospholipid molecule.
The amphipathic nature of phospholipids is responsible for the unique structure of biological membranes. In an aqueous environment, phospholipid molecules spontaneously organise themselves in a way that their hydrophobic tails are shielded from water. This means that the hydrophobic tails point inward and away from the aqueous environment, whereas the hydrophilic heads point outward. This results in the formation of two layers or lipid bilayers (Interactive 2).
Interactive 2. Three-dimensional models of a phospholipid molecule and the lipid bilayer.
In Interactive 2, you will notice that the phospholipid molecules are arranged in two layers with the tails of one layer facing the tails of the other layer. The hydrophilic heads of the inner layer face the internal aqueous environment of the cell or cell organelle, and the heads of the outer layer face the external aqueous environment.
The lipid bilayer also includes molecules of
cholesterol
(seesection B2.1.11–13)(in animal cells). These molecules are amphipathic in nature.
Lipid bilayers as barriers
Biological membranes separate the internal environment of the cell or organelle from the external environment. Lipid bilayers occur in almost all biological membranes and regulate the movement of substances.
The hydrocarbon tails of both layers extend inward to form a continuous hydrophobic interior; this has an important role in determining the permeability of the membrane (Figure 1).
As the interior of the bilayer is hydrophobic, non-polar, lipid-soluble molecules like steroids can easily pass through the lipid bilayer.
On the other hand, for the same reason, ions like Na+ cannot pass through the membrane.
Uncharged polar molecules like glucose are hydrophilic in nature. These molecules are ‘repelled’ by the cell membrane, i.e. the membrane is mostly impermeable to them.
Small, uncharged molecules can readily pass through the lipid bilayer. Thus, polar molecules like water and ethanol or non-polar molecules like oxygen and carbon dioxide can easily enter or leave cells.
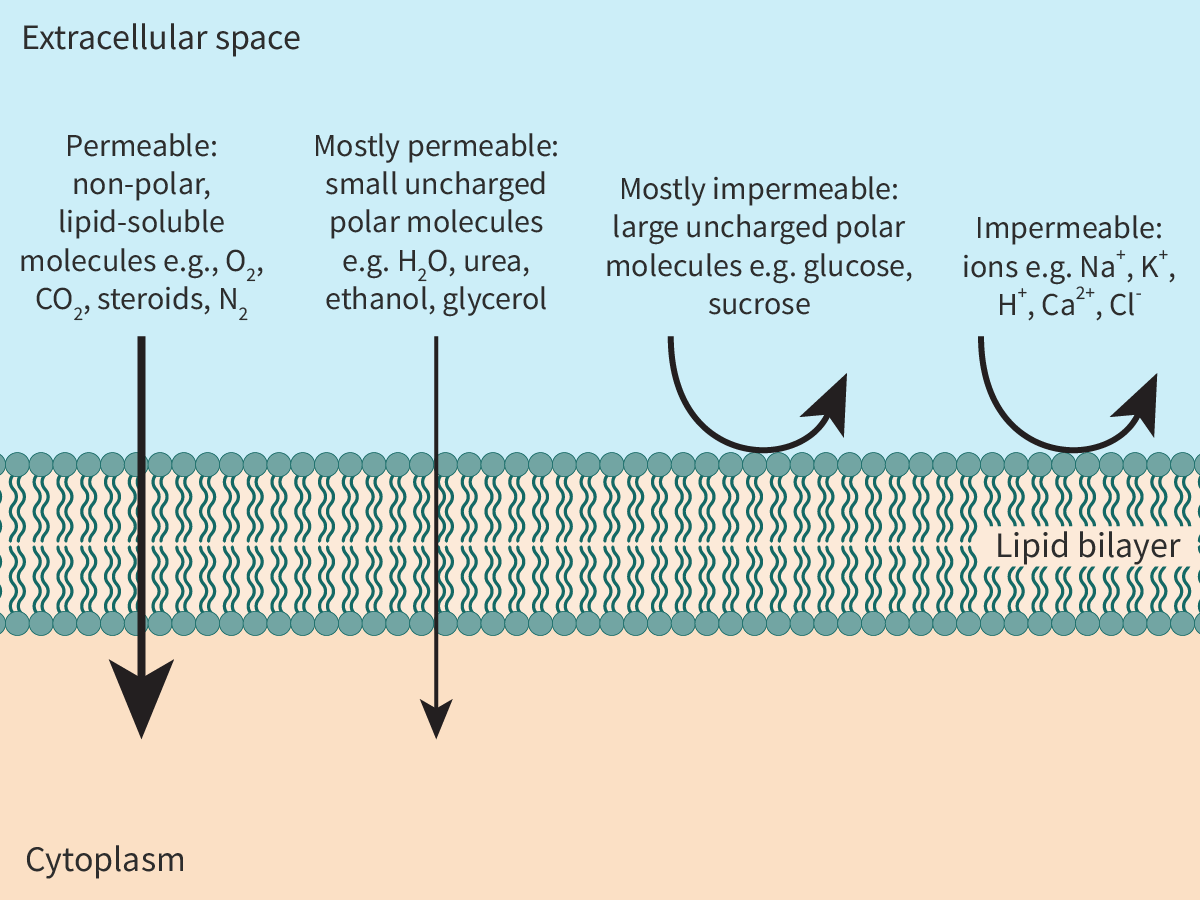
Figure 1. Permeability of the lipid bilayer.
Making connections
Just like phospholipids, fatty acids and glycerols are key components of fats (see section B1.1.8–11) too. Yet, chemically fatty acids differ from phospholipids.
The permeability of biological membranes to molecules depends on the size of the molecules and their hydrophilic/hydrophobic nature. As most of the constituents (parts) of the cell are polar or charged, biological membranes form barriers, preventing the unneeded entry or exit of these molecules from the cell.
The direction of the arrows given in Interactive 3 indicates the relative permeability of the membrane to various molecules. Drag and drop the molecules to indicate their permeability.
Interactive 3. Permeable or not?
Simple diffusion of molecules
One of the easiest ways for molecules to move across the membrane is by simple
diffusion
. Simple diffusion is the movement of molecules of a substance down a concentration gradient, i.e. from a region of where its concentration is higher to a region where its concentration is lower. Diffusion is a spontaneous process and the movement of molecules eventually results in equilibrium, i.e. in equal concentration of the molecules in both the regions. It is also a passive process, i.e. it does not involve the expenditure of energy by cells.
One example of simple diffusion is the movement of non-polar molecules like oxygen and carbon dioxide, which plays an important role in gas exchange (see subtopic B3.1). The erythrocytes or red blood cells (RBCs) transport oxygen from the lungs to the cells of the body. The oxygen diffuses from the oxygen-rich air in the alveoli down the concentration gradient to blood (erythrocytes) in the capillaries surrounding the alveoli. This oxygen is carried to tissues. In tissues, the oxygen diffuses from erythrocytes where oxygen concentration is higher to metabolically active cells where oxygen concentration is lower.
At the same time, in tissues, carbon dioxide diffuses from the cells where its concentration is higher to blood where its concentration is lower. The carbon dioxide is then carried to the lungs. In the lungs, the carbon dioxide diffuses from the blood to the alveoli down the concentration gradient. Figure 2 illustrates the process. To keep this simple, the reactions that happen within erythrocytes have not been included here.
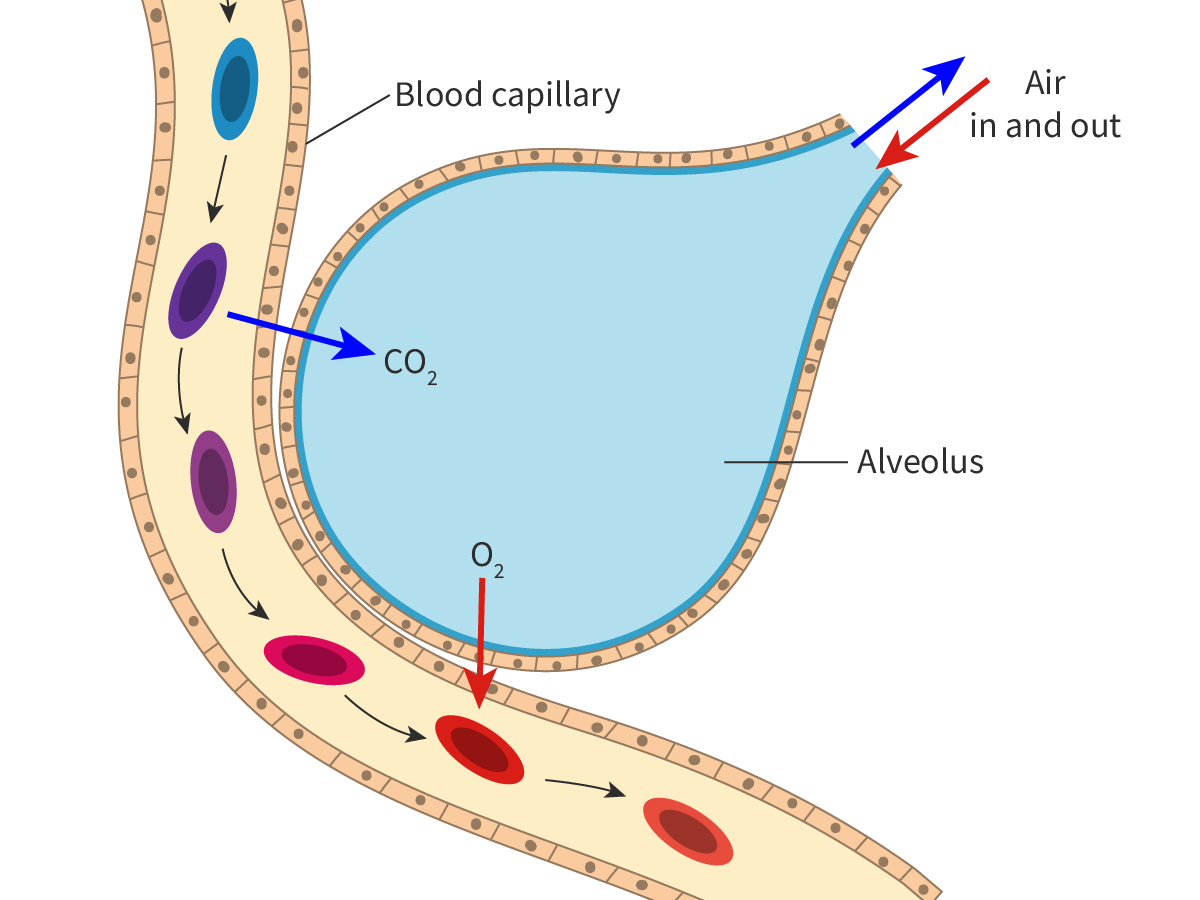
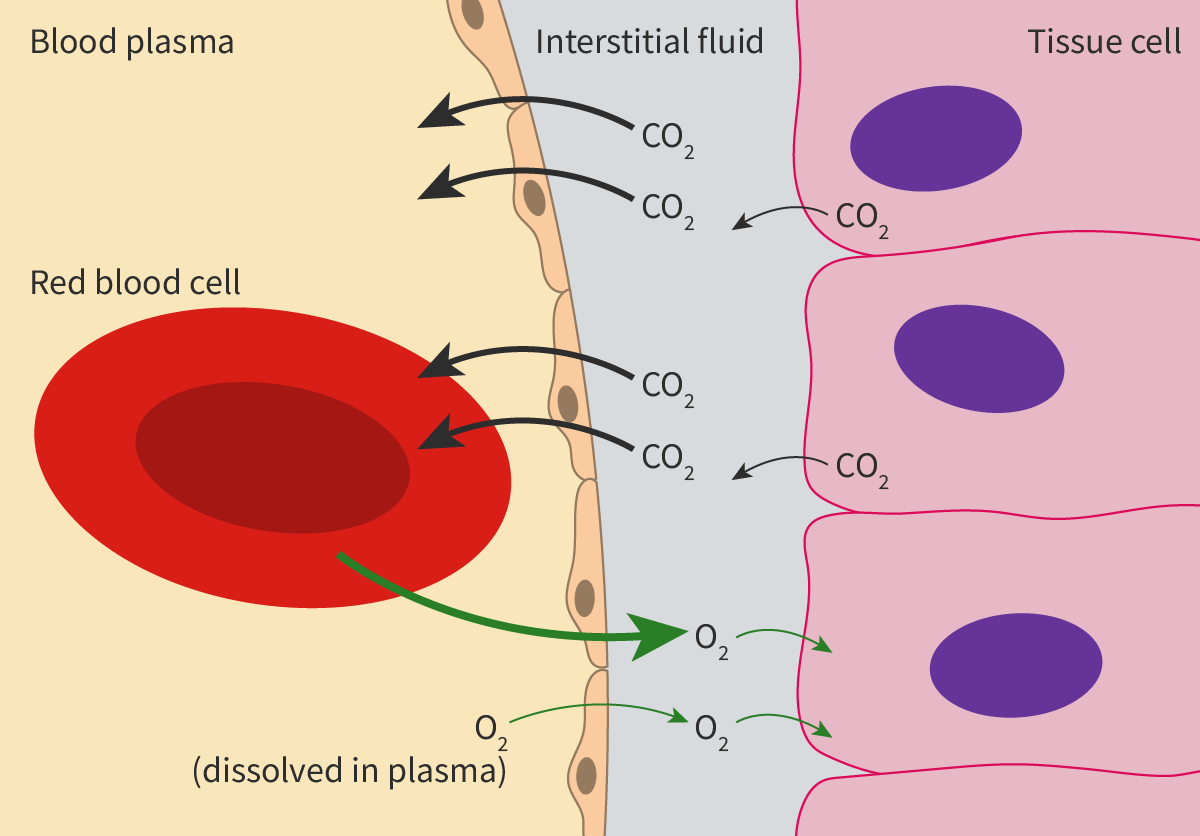
Figure 2. Diffusion of gases down the concentration gradient.
Clearly both oxygen and carbon dioxide diffuse passively and easily through lipid bilayers down their concentration gradient.
However, the hydrophobic nature of the lipid bilayer restricts simple diffusion of most molecules. Only non-polar molecules like oxygen or carbon dioxide or very small polar molecules like water or alcohol, can diffuse across membranes.
Integral and peripheral proteins
Proteins are vital components of biological membranes. Membrane proteins differ in location, structure and function.
Based on their association with the lipid bilayer, membrane proteins can be classified as:
integral proteins
peripheral proteins.
Integral proteins
As the term implies,
integral membrane proteins
are embedded in the lipid bilayer. These proteins are difficult to isolate as extraction techniques involve disrupting the bilayer. These integral proteins are amphipathic molecules. The hydrophobic regions of the integral proteins interact with the hydrophobic interior of the lipid bilayer, causing them to be embedded in the bilayer. The hydrophilic regions interact with the hydrophilic heads of the lipid bilayer or the aqueous environment.
Most integral proteins are transmembrane proteins, i.e. they extend across the membrane. Others are found only on one side of the bilayer.
Peripheral proteins
Unlike integral proteins,
peripheral proteins
are hydrophilic in nature and do not have hydrophobic regions. These proteins are normally found on the surface of the membrane and interact only with the hydrophilic regions of the integral proteins, and at times, with the hydrophilic heads of the phospholipids (Figure 1). Hence, it is easier to remove these molecules from biological membranes.
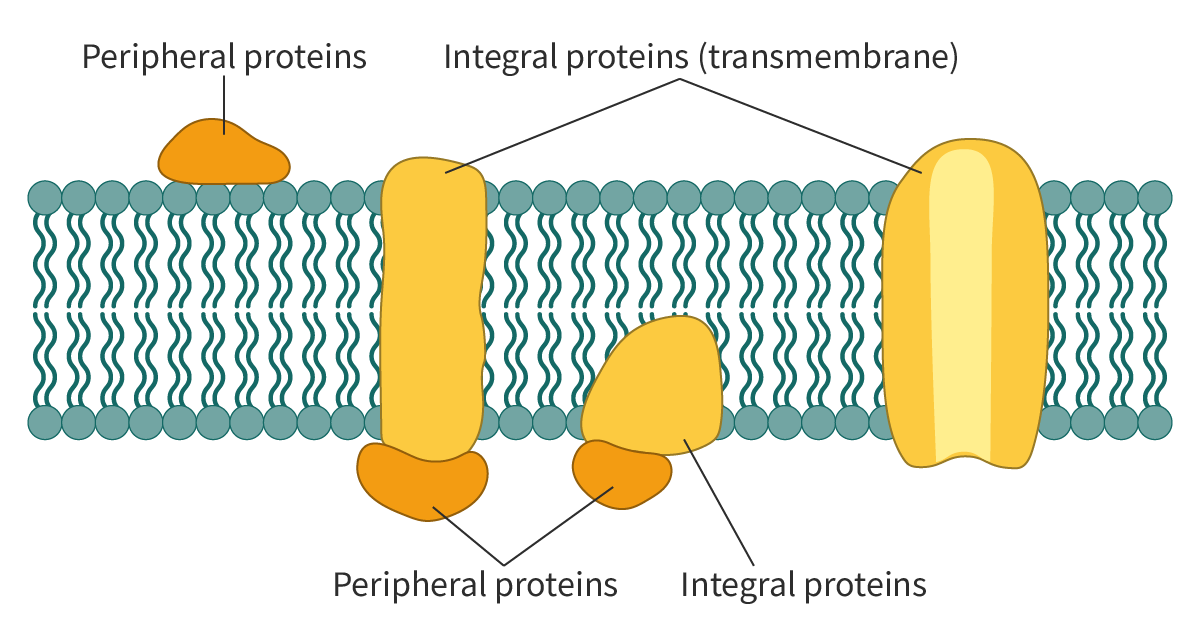
Figure 1. A schematic representation of membrane proteins.
The membrane proteins – both integral and peripheral – are asymmetrically (unevenly) oriented across the lipid bilayer.
Functions of membrane proteins
Apart from their structural role, membrane proteins have other functions, as outlined below and in Figure 2.
Transport proteins – Membrane proteins facilitate the movement of molecules in and out of the cell. These include both
channel proteins
and carrier proteins (see later in this section and B2.1.6–8). Channel proteins are transmembrane proteins that form channels or pores for the passage of molecules. Carrier proteins on the other hand undergo a conformational change to transfer the molecules from one side of the membrane to the other.
Recognition – Membrane proteins help in cell–cell recognition acting as ‘name tags’ for the cells. This is essential, especially in the functioning of the immune system, as it helps to distinguish between self and non-self cells.
Receptors – Membrane proteins act as receptors for chemical signals and are binding sites for molecules like hormones and neurotransmitters. Often, binding of these molecules triggers a chain of intracellular reactions.
Enzymes – Membrane proteins show enzymatic activity and catalyse reactions. For example, glucose-6-phosphatase is a membrane-bound enzyme found in the endoplasmic reticulum.
They can help in cell adhesion to other cells or to the environment (see section B2.1.6–8) and play a role in cell motility.
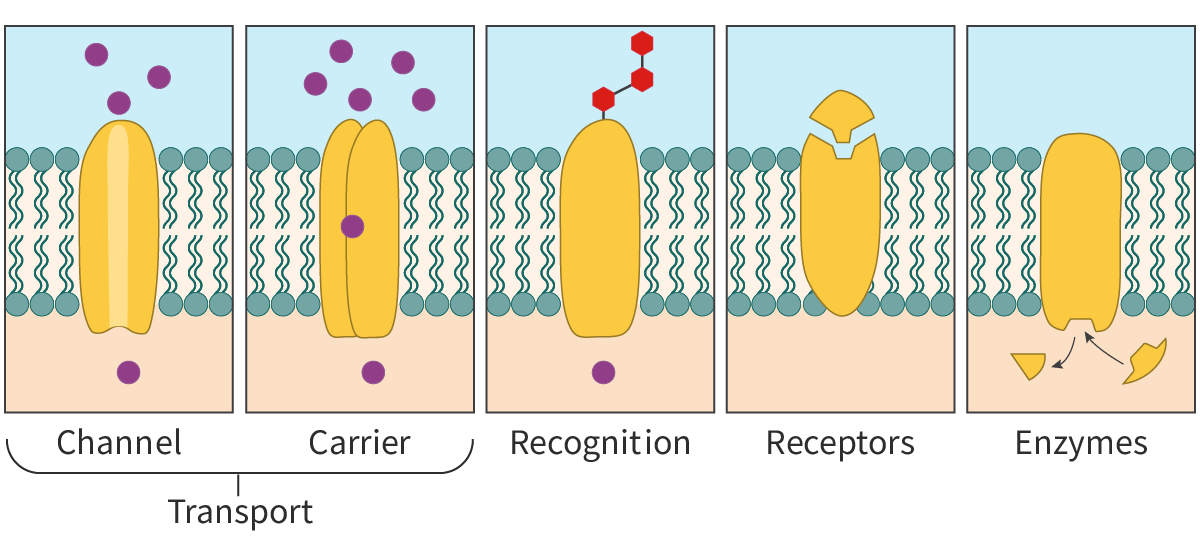
Figure 2. A simple illustration to show some of the functions of membrane proteins.
Osmosis
Place a few raisins in water and within a few hours, they swell. On the other hand, place a few grapes in a sugar solution for some time, and they become shrivelled. This is due to
osmosis
. Osmosis is the diffusion of water across a selectively permeable membrane from lower to higher
solute
concentrations. In general, the concentration of solutes tends to be higher within the cell than outside, resulting in the net movement of water into the cell.
As described in section B2.1.1–3, the permeability of biological membranes to different substances depends on the size and polar nature of the molecules. This in turn creates two regions on either side of the membrane – a region with a higher solute concentration and a region with a lower solute concentration. Water diffuses from a region of lower solute concentration (and higher water concentration) to a region with higher solute concentration (and lower water concentration) through the membrane.
Figure 3 shows osmosis of water across a selectively permeable membrane from side X to side Y. The movement of water molecules continues until the solute concentration is the same on both sides of the membrane. It is important to remember that the membrane would be impermeable to polar solutes.
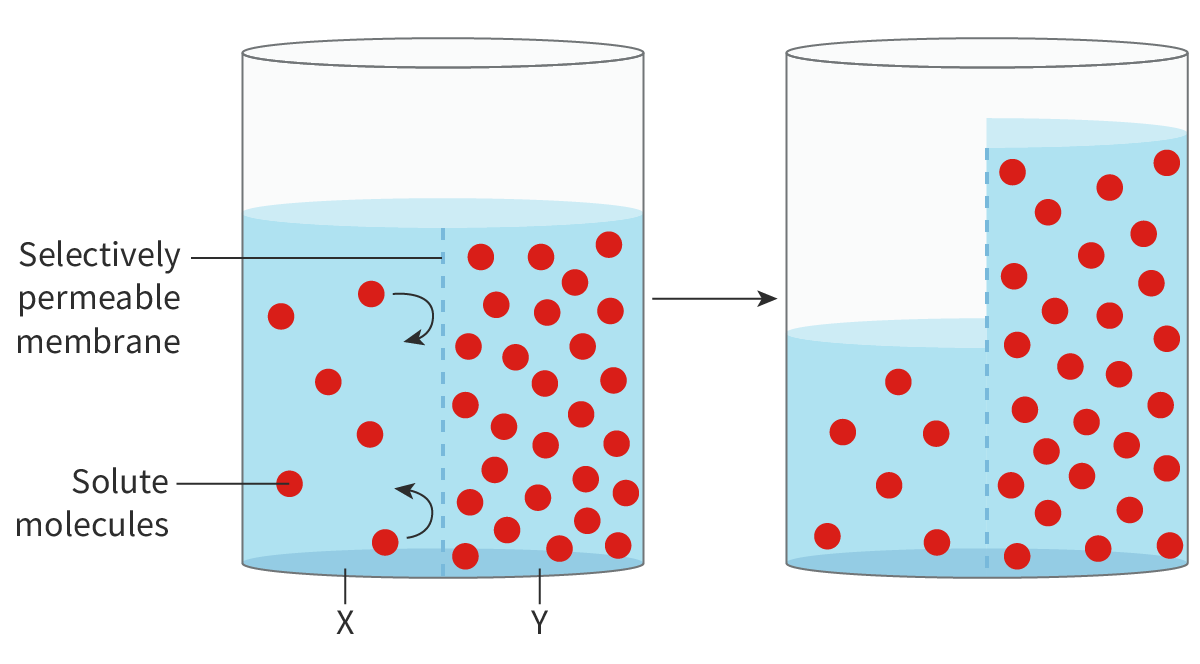
Figure 3. Osmosis.
Water always moves by osmosis from a region of higher water concentration to a region of lower water concentration. This continues until the concentration becomes the same on both sides of the membrane. Once this happens, although the random movement of water molecules continues, there is no net movement of water.
Aquaporins
The hydrophobic interior of the lipid bilayer does not allow the polar water molecules to pass through easily. Yet, the rates at which water molecules move across the cell membranes of some cells are far higher than what would be expected by mere diffusion. In 1992, Peter Agre and his colleagues discovered
aquaporins
(AQP), a type of channel protein, which explained this phenomenon.
Show or hide attributes
Scientists had been suggesting the existence of water channels back in the late 1800s. It was only when Peter Agre and his colleagues isolated the water channel proteins that these claims were verified. Scientific claims need to be supported by evidence and in this case, it was the discovery of aquaporins.
Aquaporins are integral proteins. A typical AQP molecule is a
tetrameric protein
composed of four monomeric subunits(see quaternary structure,section B1.2.11). Each subunit has a water channel, so, an aquaporin molecule has four identical water channels (Figure 4).
The water channels are lined with specific hydrophilic side chains (of amino acid residues) which allows the passage of water molecules but not of ions. The water molecules pass in a single file through the channels, but, even then, several billion molecules pass through a single channel at one time.
It is important to remember that aquaporins are bidirectional, i.e. water can flow in either direction: into the interior or out to the exterior of the cell depending on the concentration gradient.
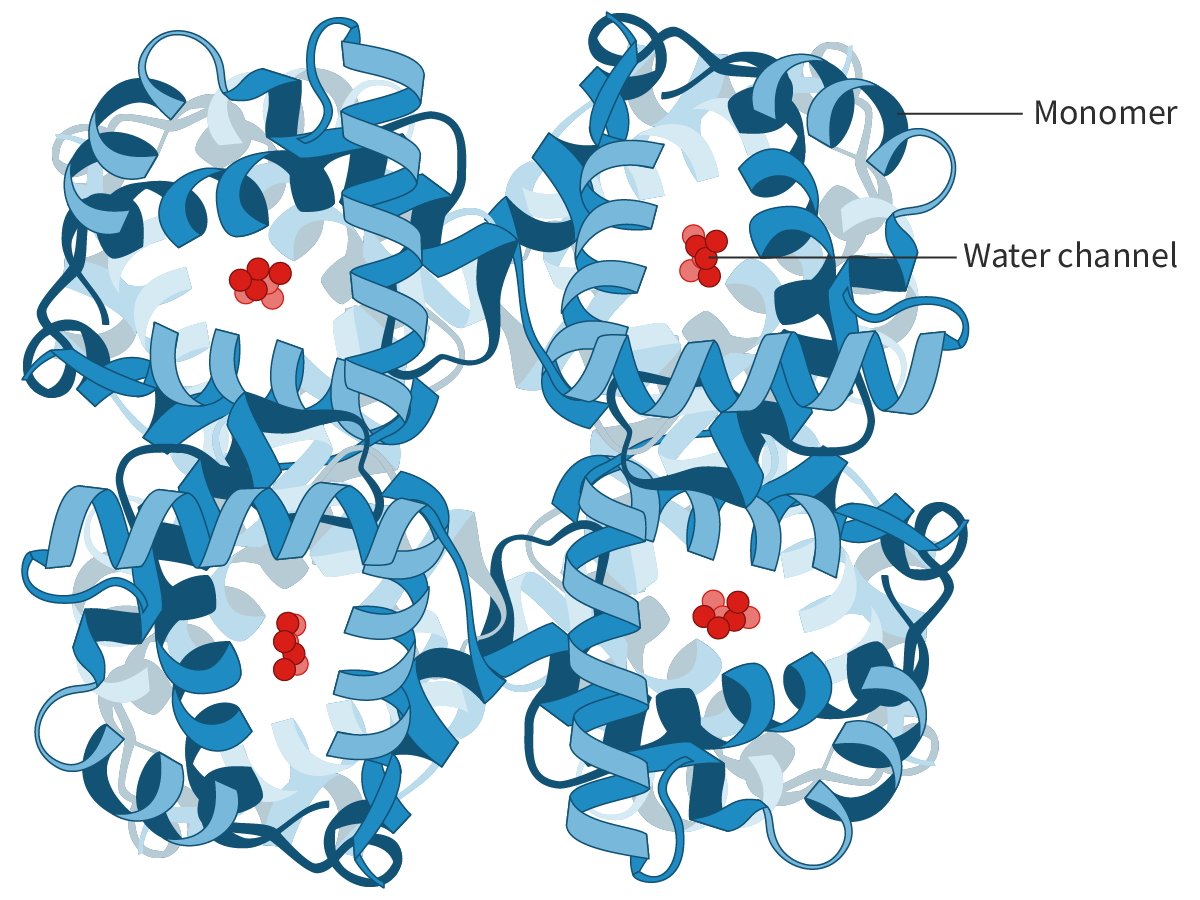
Figure 4. An aquaporin tetramer.
Aquaporins thus permit the rapid movement of water in and out of the cell by forming hydrophilic channels that span across the membrane. The volume of water that needs to be transported across the cell membranes determines the number of aquaporins. For example, the cells of the kidney reabsorb water and hence, have a higher concentration of aquaporins.
Facilitated diffusion
Diffusion is the movement of solutes down their concentration gradient. However, even down the concentration gradient, the size or the polar nature of most molecules prevents them from crossing the cell membrane. The movement of these molecules is mediated by proteins which could be either carrier or channel proteins.
This type of movement is called
facilitated diffusion
, because:
the movement of the molecules is down the concentration gradient
the movement is assisted (facilitated) by transport proteins.
Facilitated diffusion aided by channel proteins
Channel proteins, as the term implies, are transmembrane proteins that assemble to form channels for the passage of polar molecules. One example are ion channels, the tiny pores of which act as pathways for ions like Na+ and K+. These channels are highly selective and often, different channels are needed for the transport of different ions.
The selectivity of the ion channels is due to:
the binding sites of the hydrophilic amino acid side chains lining the channel being highly ion-specific
the size of the pore acting as a size filter.
Most channels open or close in response to specific stimuli, such as:
changes in voltage across the membrane or voltage-gated channels
binding of small molecules to the channel proteins or
ligand
-gated channels
mechanical forces like pressure.
Hence, ion channels in facilitated diffusion are gated (see section B2.1.14–16). When the gates are open, the ions pass through the pore down the concentration gradient. On the other hand, in a closed state, the pore is plugged, preventing the passage of the ion (Figure 1).
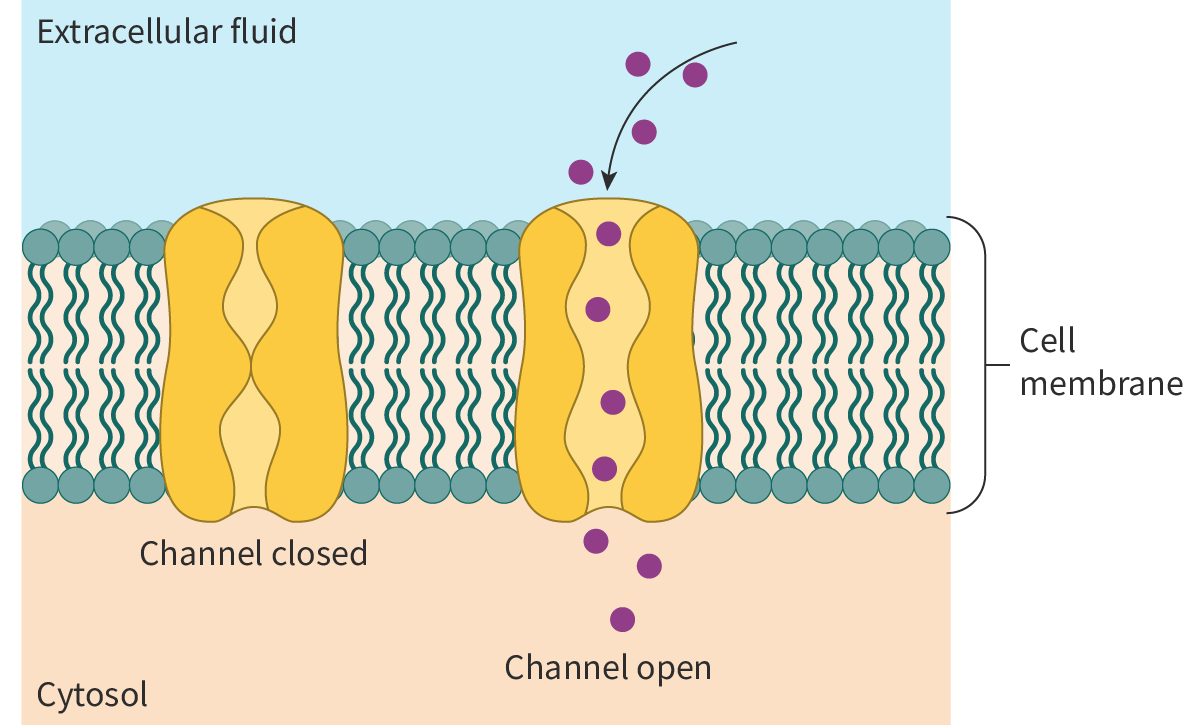
Figure 1. Facilitated diffusion via ion channels.
Porins are another example of channel proteins. These channels tend to be less specific and are larger.
Facilitated diffusion aided by carrier proteins
Like channel proteins, carrier proteins are transmembrane transport proteins that play an important role in facilitated diffusion. However, the mechanism is very different. The carrier protein binds to the solute molecules (molecules to be transported), undergoes a conformational change and transfers the molecules to the other side of the membrane (Figure 2). Carrier proteins have sites specific for the solute or class of solutes to be transported and hence are highly specific. One example is the GLUT or glucose transporter, a carrier protein that helps in the transport of glucose into the red blood cell (RBC) down its concentration gradient.
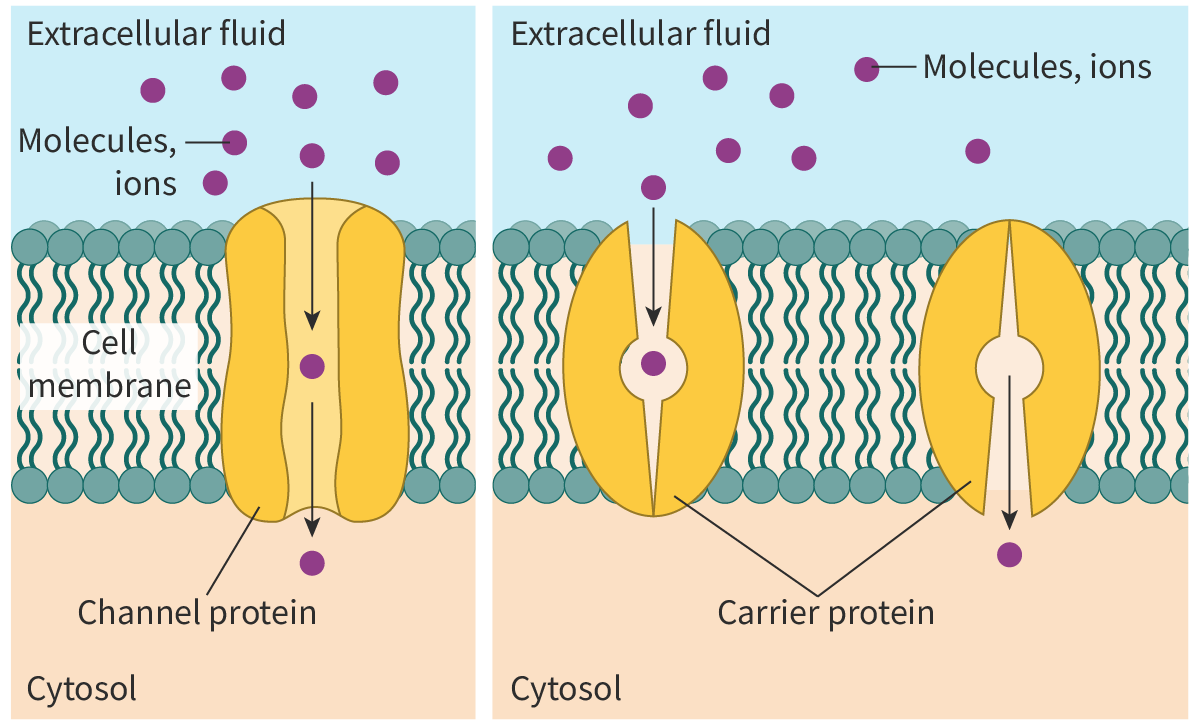
Figure 2. Facilitated diffusion with the help of carrier proteins.
Video 1 shows an animation of facilitated diffusion mediated by channel and carrier proteins.
Video 1. Facilitated diffusion aided by channel and carrier proteins.Active transport and pump proteins
Imagine a waterfall. Water cascades down. On the other hand, if water has to be transported to the top of a building, it has to be pumped to overcome the force of gravity. A similar situation exists in cells: when ions or molecules have to be moved against their concentration gradient they need to be pumped.
Active transport
comes into play when molecules need to be transported from a region of their lower concentration to a region of their higher concentration, i.e. against their concentration gradient. As the transport is ‘uphill’, energy is required. In other words, the transport of the molecules is coupled with an energy-releasing or
exergonic
reaction like the breakdown ofATP.
The transport proteins used for active transport are often called pumps as they move the molecules against their concentration gradient (Figure 3).
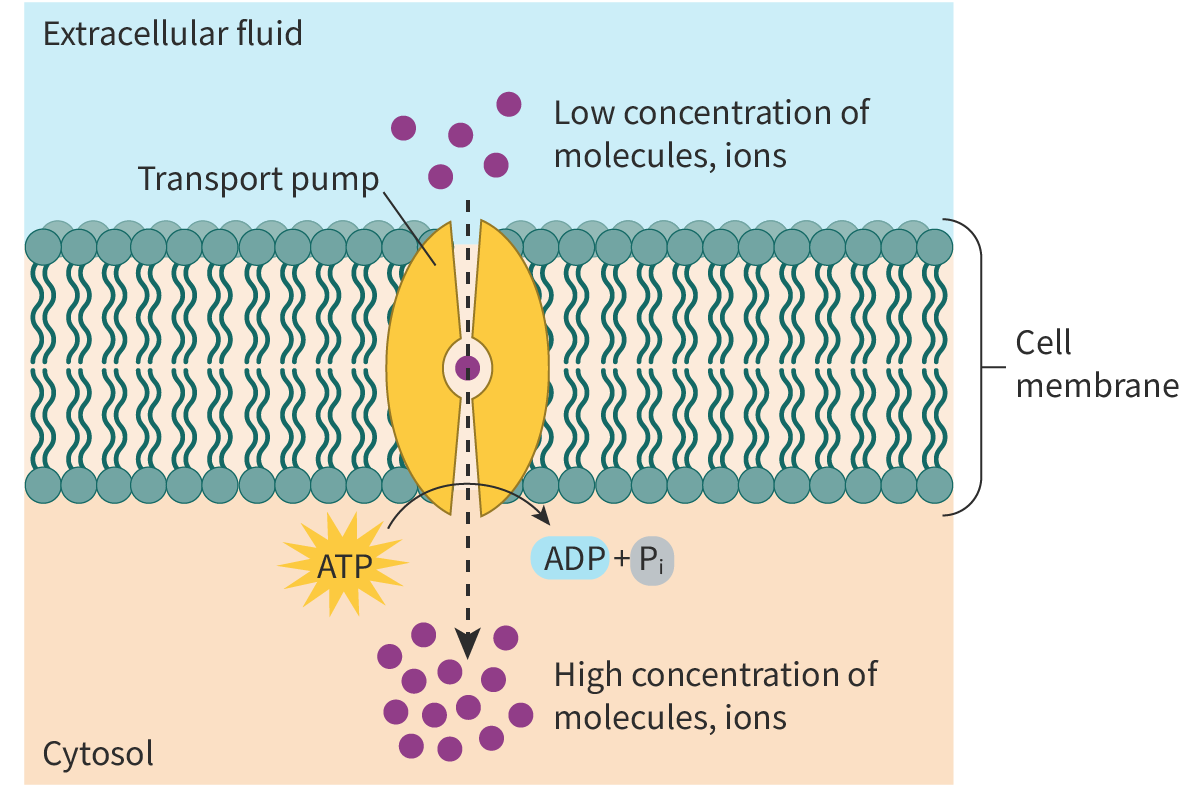
Figure 3. Active transport.
Active transport helps to:
take up essential nutrients – an example is the uptake of glucose from the lumen of the intestine to the epithelial cells lining the small intestine (see section B2.1.14–16)
remove secretory or waste materials from the cell into the extracellular fluid
maintain the right concentrations of ions in the cells; for example, active transport helps red blood cells (RBCs) maintain their internal sodium and potassium levels.
Study skills
The mode of transport of glucose changes depending on the concentration gradient and the transport proteins involved. Glucose is transported across the cell membrane using transport proteins like GLUTs or sodium-dependent glucose cotransporters (SGLTs) (see section B2.1.14–16). However, GLUTs help in facilitated diffusion whereas SGLTs help in active transport.
Types of active transport
There are two main types of active transport.
Direct active transport is where the energy released by an exergonic reaction like the breakdown of ATP is used to directly transport molecules across the cell membrane (see section B2.1.14–16). As energy is derived by the hydrolysis of ATP, these transport proteins are called ATPases or ATPase pumps.
Indirect active transport or cotransport, where the movement of one solute down its concentration gradient drives the movement of the second solute against its concentration gradient (see section B2.1.14–16).
Video 2 outlines how active transport differs from diffusion.
Video 2. Active transport in cells.
Interactive 1 shows the different forms of membrane transport. Drag and drop to match the terms with the images.
Interactive 1. Membrane transport.
Selectivity in membrane permeability
It is evident that biological membranes are designed in a way to let some molecules in and keep out others.
The movement of molecules by simple diffusion is based on two factors: the size of the molecules and their hydrophobic or hydrophilic nature. In other words, the permeability of the membrane depends on the physical properties of the molecules. Any molecules that fit these criteria, irrespective of whether they are useful or harmful, pass through the cell membrane. Thus, in the instance of simple diffusion, permeability of the cell membrane is not selective.
However, facilitated diffusion and active transport involve proteins that transport molecules from one side of the membrane to the other. These transport proteins, whether channel proteins or carrier proteins, exhibit selectivity as they recognise specific molecules or classes of molecules. For example:
Gated ion channels like calcium-specific ion channels in muscle cells regulate the movement of calcium ions alone.
Carrier proteins are highly specific. For example, GLUT (glucose transporter) recognises and binds only to glucose and a few other monosaccharide molecules.
The
selective permeability
of the membrane is hence due to facilitated diffusion and active transport.
Glycolipids and glycoproteins
In addition to phospholipids and proteins, most membranes also consist of small amounts of carbohydrates. These carbohydrates are either linked to lipids – forming glycolipids, or linked to proteins – forming glycoproteins.
Glycolipids
The covalent bonding of carbohydrates to lipids results in glycolipids. Vital parts of the cell membranes, glycolipids are amphipathic molecules, often restricted to the external surface of the cell membrane. The carbohydrate groups of these molecules are polar and extend into the extracellular environment, whereas the non-polar lipid component lies embedded in the bilayer.
Based on their structure, glycolipids are classified into
glycoglycerolipids or glycerol-based lipids
glycosphingolipids or derivatives of sphingosine; examples include cerebrosides and gangliosides.
Glycolipids contribute to membrane stability as they form hydrogen bonds with the water molecules surrounding the cell.
Glycoproteins
The covalent bonding of oligosaccharides (short carbohydrate chains) to the protein molecules results in the formation of glycoproteins. The carbohydrate groups of the glycoproteins often protrude (stick out) into the extracellular environment.
Functions of glycolipids and glycoproteins
Cell recognition: Glycolipids and glycoproteins play an important role in cell recognition. They act as ‘markers’ on the cell surface and help cells of the body recognise each other. They also help cells of the immune system to recognise foreign cells.
Cell adhesion: Both glycolipids and glycoproteins help cells to attach and bind to other cells to form tissues. Cell-adhesion molecules or CAMs (see section B2.1.17) are cell-surface glycoproteins that play an important role in cell adhesion.
Cell signalling: They act as receptors for enzymes and other molecules helping in cell signalling, i.e. receiving and transmitting chemical signals.
Figure 1 shows how the type of antigens (glycolipids and glycoproteins) present on the surface of the red blood cell membranes determines blood groups.
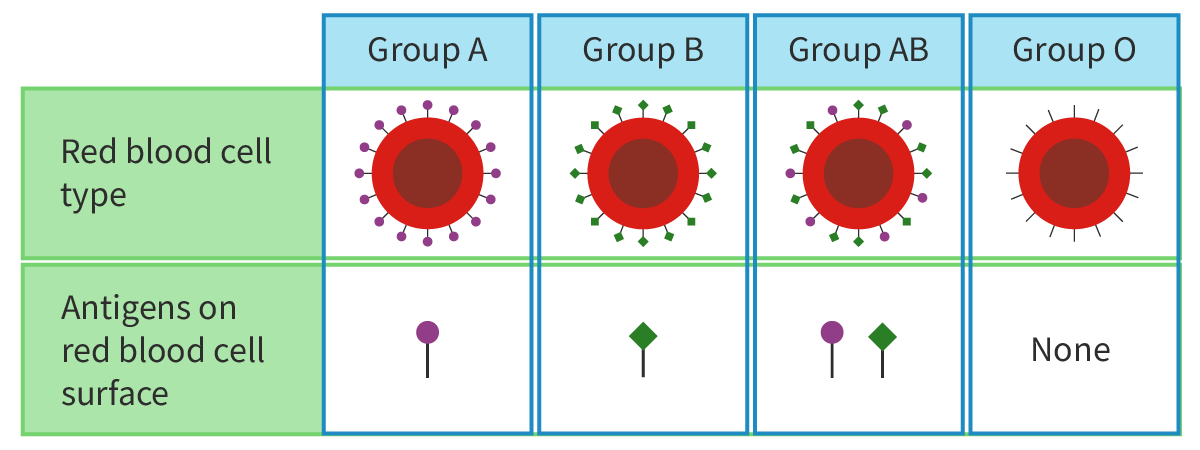
Figure 1. Blood groups are determined by the type of antigens (glycolipids and glycoproteins) present on the surface of the red blood cell membranes.
Glycocalyx: The glycocalyx (Figure 2) is the sticky layer formed by the carbohydrate groups of the glycolipids and glycoproteins that protrude from the cell surface. The glycocalyx in addition to its roles in cell signalling, cell adhesion and cell–cell recognition, helps in protecting the cell surface.
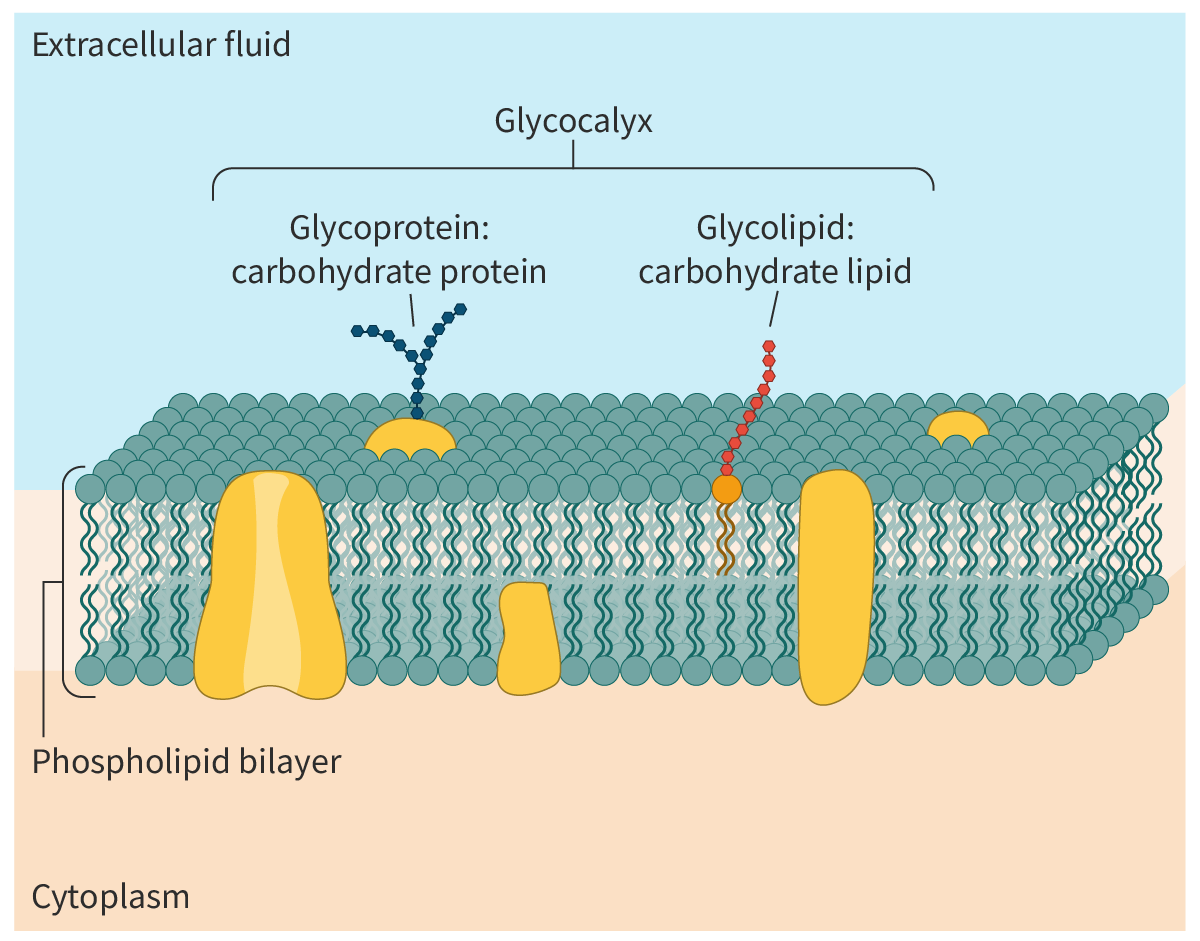
Figure 2. Glycolipids and glycoproteins.
Fluid mosaic model
The fluid mosaic model of membrane structure was proposed by Singer and Nicolson in 1972 and describes the arrangement of the lipids and proteins. The model (Interactive 1) states that:
the lipid bilayer is fluid – the fluidity of the membrane depends on the nature of the fatty acids in the phospholipid molecules and the amount of cholesterol (see section B2.1.11–13)
the proteins (both integral and peripheral) are embedded in the fluid bilayer which resembles a mosaic.
The fluid nature of the membrane means that most of the lipids and proteins are able to move laterally, parallel to the membrane surface. Thus, according to the fluid mosaic model, the membrane is organised as a mosaic of proteins present within a fluid lipid bilayer.
The fluid mosaic model provides a framework to understand membrane structures; however, research continues to enhance our understanding and explain new findings.
Membrane fluidity depends on the fatty acid composition of the phospholipids
All membrane lipids, whether phospholipids or glycolipids, have two long fatty acid chains. These fatty acid chains are hydrophobic in nature.
The fatty acids of the membrane lipids vary in length and in the number of double bonds.
The number of carbon atoms in the fatty acid side chains of membrane lipids normally ranges from 16 to 20, resulting in variations in length of the fatty acids.
The fatty acids also vary in their degree of saturation. Some fatty acids – like palmitic acid and stearic acid – seen in the membrane lipids are saturated (section B1.1.8–11), i.e. there are no double bonds between the carbon atoms. Others – like oleic acid – are unsaturated with one or more double bonds between the carbon atoms.
The saturated fatty acids, with their higher melting points, provide stability to the membrane. The unsaturated fatty acids, with their lower melting points, ensure the fluidity of the membrane (Figure 1).
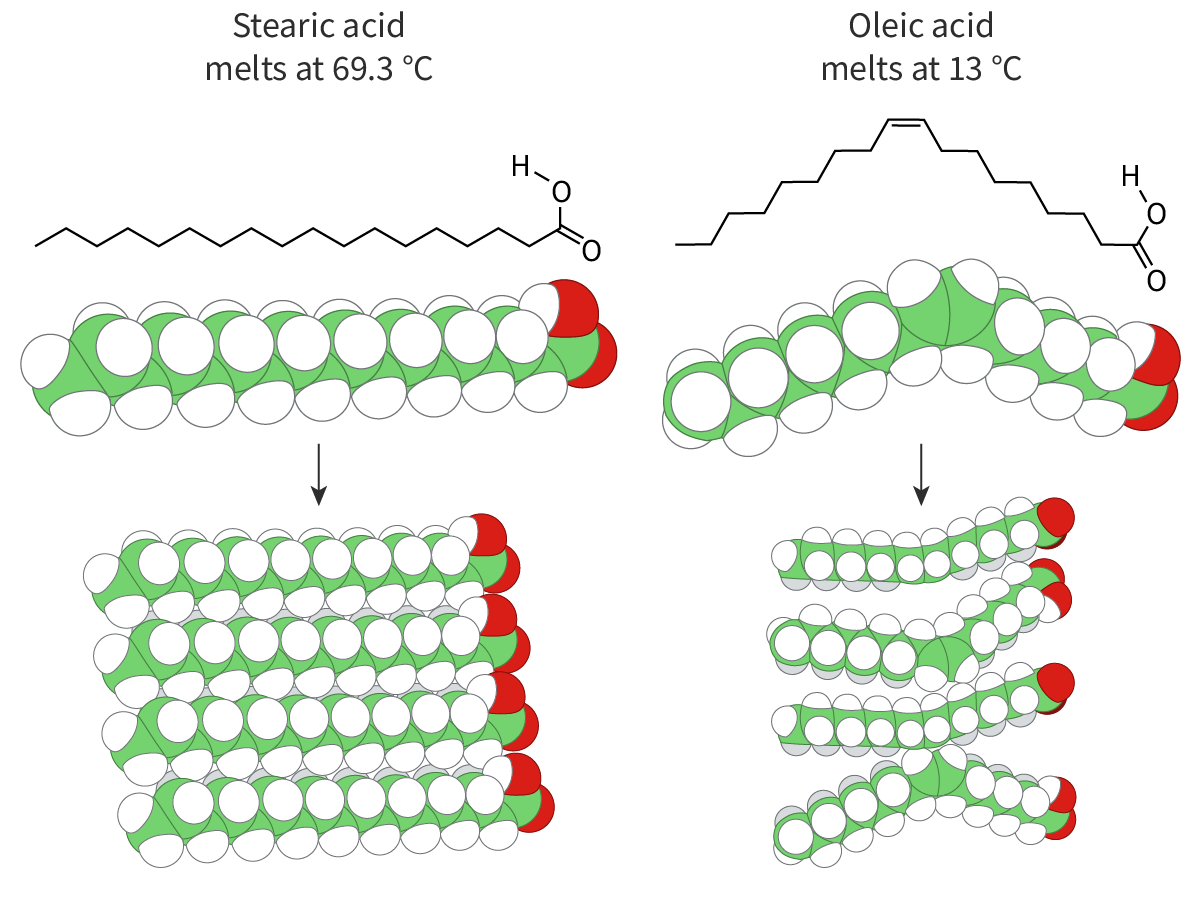
Figure 1. Saturated and unsaturated fatty acids.
You can see in Figure 1 that unsaturated fatty acids have kinks in their chains whereas the ones with saturated fatty acids do not. As their fatty acid chains lie parallel to each other, lipids with saturated fatty acids fit together snugly, making the membrane denser and more rigid. On the other hand, the kinks prevent membrane lipids with unsaturated fatty acids from packing together closely, maintaining fluidity.
What happens when the temperature drops? At low temperatures, the phospholipid molecules come closer together, making the membrane more ‘gel-like’ and decreasing fluidity. This is where the ‘space’ created due to the kink in the tails becomes important – it prevents the molecules from packing too closely together and helps to maintain the membrane fluidity. Thus, saturated fatty acids freeze more easily than unsaturated fatty acids.
Cold-blooded organisms like frogs adapt to lower temperatures by increasing the proportion of unsaturated fatty acids in their membranes thereby regulating the fluidity. A similar example is seen in hibernating animals. During hibernation, as the body temperature of the mammal drops, the proportion of unsaturated fatty acids in the membrane phospholipids increases.
Membrane fluidity depends on cholesterol in animal cells
Cholesterol plays an important role in the fluidity of biological membranes. Cholesterol is an amphipathic steroid (see section B1.1.12–13). The hydrophobic region comprises four steroid rings and a hydrocarbon side chain; the hydrophilic region is a polar hydroxyl group (Figure 2). Found in one of the two layers, the hydrophilic and hydrophobic regions of a cholesterol molecule interact with the corresponding hydrophilic and hydrophobic regions of adjacent phospholipid molecules. These interactions hold the phospholipid molecules together.
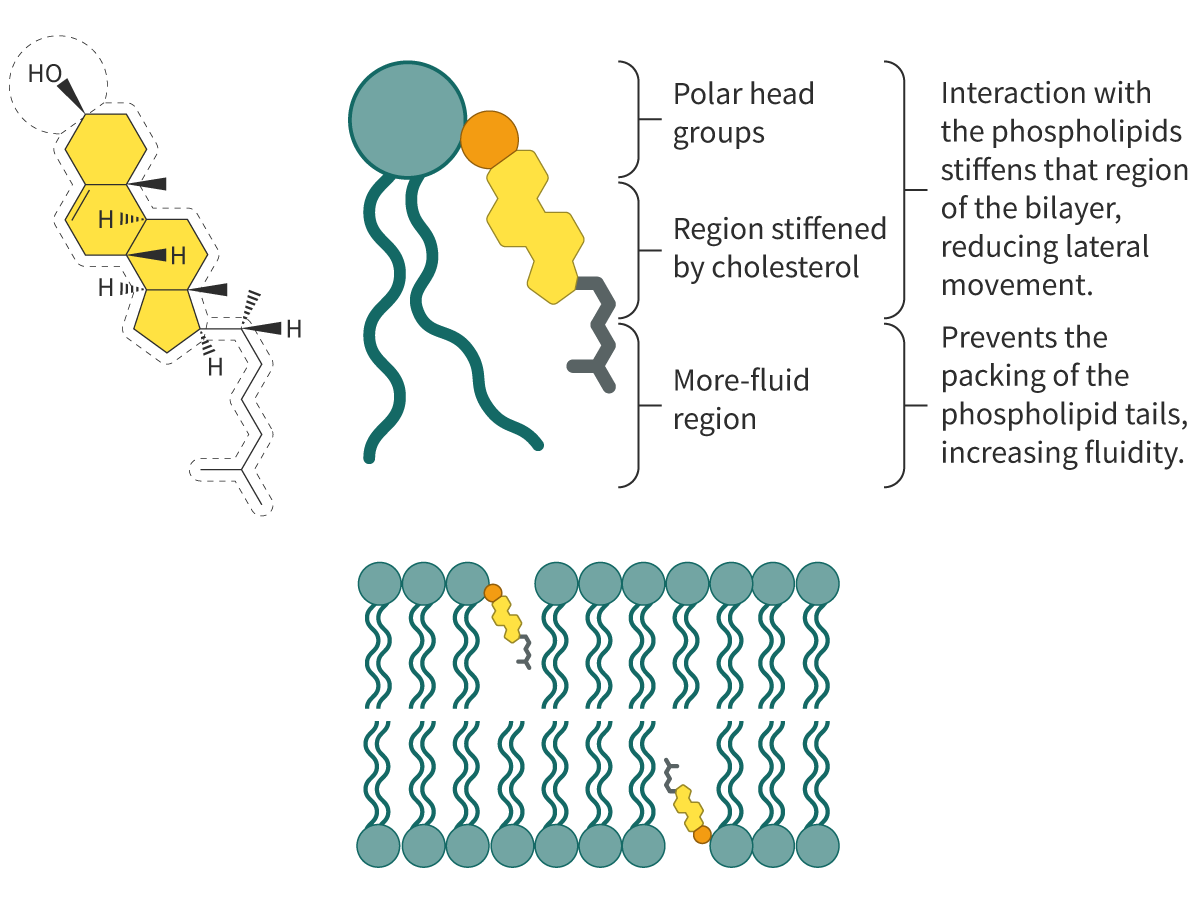
Figure 2. Influence of cholesterol on membrane fluidity.
The insertion of cholesterol molecules into the lipid bilayer works as follows:
At low temperatures, the ‘inserted’ cholesterol prevents the fatty acid chains of the phospholipids from fitting closely together, preventing the tendency of the membranes to freeze.
At high temperatures, cholesterol stabilises the membrane and reduces fluidity.
It decreases the permeability of the membrane to ions and molecules.
Membrane fluidity and formation of vesicles
Often large amounts of material need to be transported in and out of the cell. The two bulk transport mechanisms utilised by cells are
exocytosis
and
endocytosis
. Both of these processes require energy and hence are active transport mechanisms.
Endocytosis
Endocytosis (endo – inside; cytosis – transport mechanism) is a bulk transport mechanism by which particles are moved into the cell. The cell membrane progressively invaginates and eventually engulfs the particles (to be taken in). The membrane then pinches off to form a vesicle with the ingested particles (Interactive 1).
Interactive 1. Endocytosis. |
The ingestion of large solid particles is called
phagocytosis
(cellular eating); the ingestion of liquids is called
pinocytosis
(cellular drinking).
Phagocytosis is seen in white blood cells (WBCs) and in unicellular organisms like Amoeba.
In the case of WBCs, projections of the membrane called pseudopodia gradually surround the foreign particles or microorganisms. Eventually the pseudopodia meet and engulf the particle resulting in a vesicle called a phagosome (food vacuole). The phagosome now fuses with a lysosome. The digestive enzymes of the lysosome (see section A2.2.4–6), digest the particle, releasing nutrients. Within the phagosome, the particle is digested. Video 2 shows phagocytosis of foreign particles by neutrophils, a type of WBC.
Video 2. Phagocytosis. |
During pinocytosis, the cell takes in small amounts of extracellular fluid. Unlike the large vesicles formed during phagocytosis, the vesicles formed are smaller.
Another type of endocytosis is receptor-mediated endocytosis (see section B2.2.7–9).
Exocytosis
Exocytosis (exo – external; cytosis – transport) is the reverse of endocytosis and involves the bulk transport of material to be secreted or excreted out of the cell. The material to be removed from the cell is enclosed in vesicles. The vesicles move to the plasma membrane and fuse with it, discharging its contents to the exterior (Interactive 2).
One example of exocytosis is the glycolipids produced by the endoplasmic reticulum and modified in the Golgi apparatus. The vesicles released by the Golgi apparatus fuse with the plasma membrane discharging their contents to the outside. Many other materials like enzymes, peptide hormones and antibodies are secreted by the cell via exocytosis.
Waste products and undigested food material are also excreted by the cell via exocytosis.
Interactive 2. Exocytosis. |
Endocytosis and exocytosis have opposite effects which are not restricted to the movement of materials. In exocytosis, as vesicles fuse with the plasma membrane, lipids and proteins are added. In endocytosis, the reverse happens during the invagination and pinching off of the plasma membrane to form vesicles.
Membrane fluidity plays an important role in the structural stability of vesicles during their formation and fusion in endocytosis and exocytosis.
Dictionary
Gated ion channels in neurons
You may recall that ion channels (see section B2.1.6–8) are transmembrane proteins present on the cell membrane that form pores for the movement of ions across the membrane, down their concentration gradient. The physical structure of these channels is such that they demonstrate selectivity, meaning that only specific ions can pass through them.
These channels are usually gated, that is, they open or close in response to stimuli. Depending on the type of stimuli, they are classified as
voltage-gated channels
ligand-gated channels
mechanically gated channels (which respond to mechanical cues such as sound waves and vibrations).
In this section, you will learn about two types of gated channels that are seen in neurons.
Resting membrane potential
One common example of voltage-gated ion channels is seen in nerve signalling.
Neurons are cells of the nervous system that transmit information in the form of electrical impulses. Even at rest (i.e. when the neuron is not conducting an impulse), there is a potential difference across the nerve cell membrane of −70 mV, which means that the inside of the neuron is more negatively charged than the extracellular environment. This is the
resting membrane potential
An electrochemical gradient exists across the nerve cell membrane with a higher concentration of potassium ions inside the cell. There is more about this insection C2.2.1–4.
Action potential and the role of the voltage-gated channels in neurons
Neurons are inherently excitable. A stimulus causes the generation of an electrical impulse. An impulse is a temporary reversal in the potential difference. This change in potential is due to the opening of voltage-gated sodium channels and voltage-gated potassium channels. Both these channels are highly specific and remain closed during the resting state. Let us learn how these channels work.
A stimulus causes the ‘activation gate’ of the voltage-gated sodium channels to open first. Sodium ions diffuse rapidly into the neuron. The entry of the sodium ions causes the interior of the neuron to be more positively charged than the exterior (
depolarisation
), generating an action potential. The
action potential
travels down the nerve fibre (seesection C2.2.1–4).
Almost immediately, the voltage-gated sodium channels close and the voltage-gated potassium channels open. Potassium ions diffuse out of the neuron down their concentration gradient and the interior of the neuron becomes less positive (
repolarisation
). Eventually, the potassium channels close. The resting membrane potential is now re-established with the help of the sodium–potassium pump, as describedlater in this section.
It is important to note that the gated channels would open or close only when the voltage reaches a certain minimum value called the threshold value. In addition, voltage-gated channels undergo channel inactivation where an inactivation particle blocks the channel pore (Figure 1). During this time, the channels do not open at all – it is like a padlock on a gate!
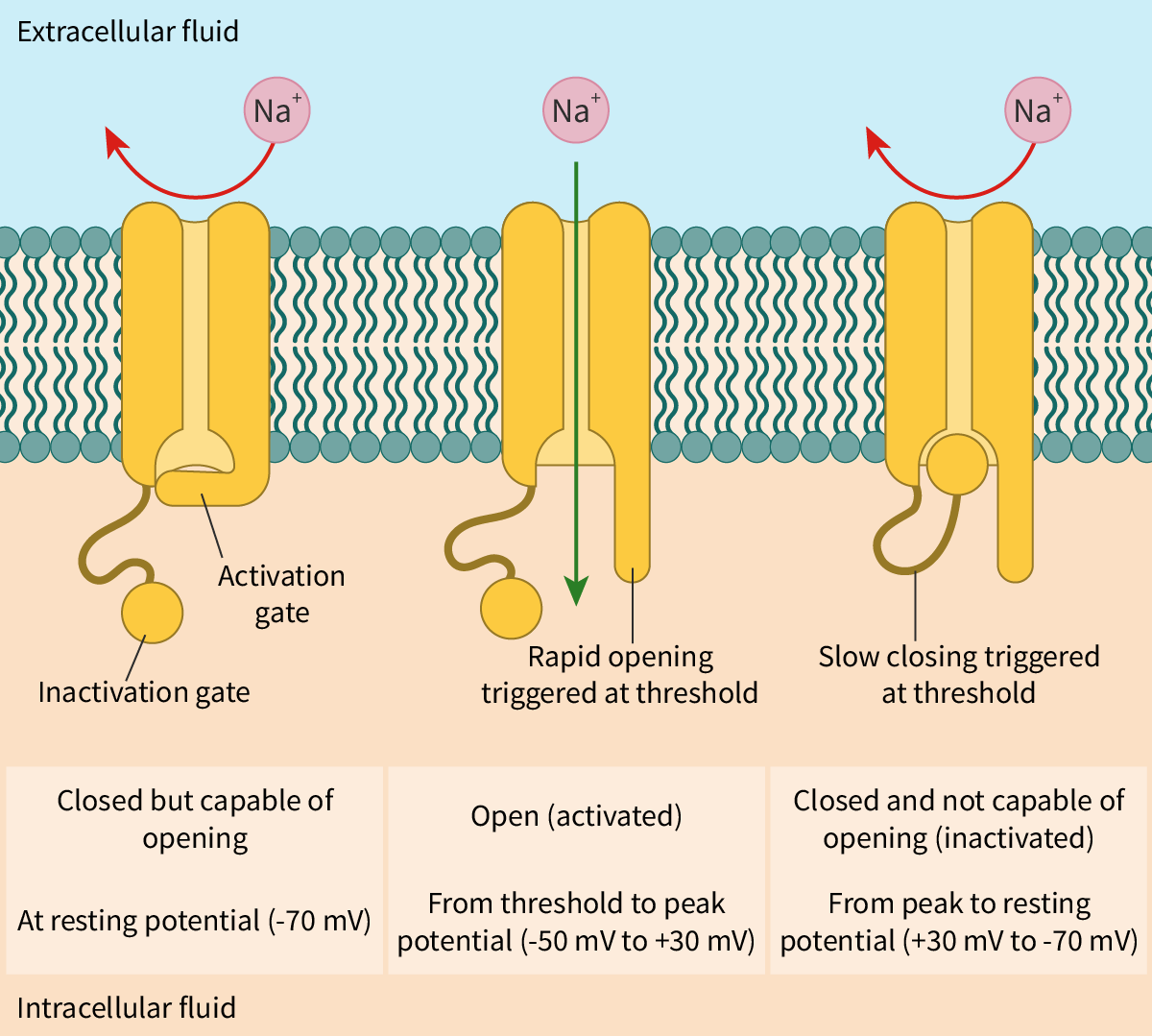
Figure 1. Opening and closing of the voltage-gated sodium channel.
Nicotinic acetylcholine receptor: a ligand-gated channel
Ligand-gated channels are ion channels that open when a ligand binds to the transmembrane protein (of the ion channel). When the ligand is a
neurotransmitter
, the ion channel is called a neurotransmitter-gated ion channel.
Acetylcholine is an excitatory neurotransmitter. Nicotinic acetylcholine receptors (nAchR) are ligand-gated ion channels present at skeletal neuromuscular junctions (Interactive 1). The binding of acetylcholine molecules results in a conformational change that opens the channel. Sodium ions now diffuse down their concentration gradient resulting in the interior of the cell becoming more positive (depolarisation). Within a millisecond, the enzyme cholinesterase breaks down acetylcholine, leading to closure of the ion channels.
Nicotine is a component of cigarettes. Like acetylcholine, nicotine can activate these channels, hence the term ‘nicotinic’ acetylcholine receptors.
Depolarisation is followed by opening of voltage-gated potassium channels and exit of potassium ions resulting in repolarisation. Soon the voltage-gated potassium channels close and resting membrane potential is restored.
Interactive 1. Nicotinic acetylcholine receptor. |
Show or hide attribute
In science, the evidence collected by direct observation or experimentation is used to support claims. The functioning of gated channels in response to changes in voltage or binding of different ligands including medicines and toxins are ongoing areas of research. The evidence collected would help scientists support or refute current hypotheses. The evidence could also be used by scientists to understand how the ion channels function and how their behaviour can be manipulated for treating diseases like epilepsy or chronic pain.
Direct active transport: the sodium–potassium pump
The sodium–potassium (Na+/K+) pump is found in the cell membranes of all animal cells and involves active transport. The Na+/K+ pump is an enzyme that generates energy by the breakdown (hydrolysis) of ATP. For this reason, the Na+/K+ pump is called Na+/K+ ATPase. The energy released in the process is used to drive the transport of sodium and potassium ions against their concentration gradient.
Most animal cells – including neurons – have a high intracellular concentration of K+ and a low intracellular concentration of Na+, creating an electrochemical gradient. This asymmetric distribution of ions is in part due to the Na+/K+ pump, with the active transport of K+ into the cells and Na+ out of the cells.
Working of the Na+/K+ pump
The Na+/K+ pumps are transmembrane pumps with three binding sites for sodium and two for potassium (Figure 2).
Initially, the Na+/K+ pump is open to the inside of the cell in a way that the sodium ions bind to all three of its binding sites.
The binding of sodium ions triggers the hydrolysis of ATP to ADP and a phosphate group. The latter attaches to the pump resulting in a conformational change. The pump now opens to the exterior releasing the sodium ions.
At the same time, potassium ions attach to both binding sites. This causes the phosphate group to detach from the pump.
The pump undergoes a conformational change to regain its original form and once again opens to the interior of the cell.
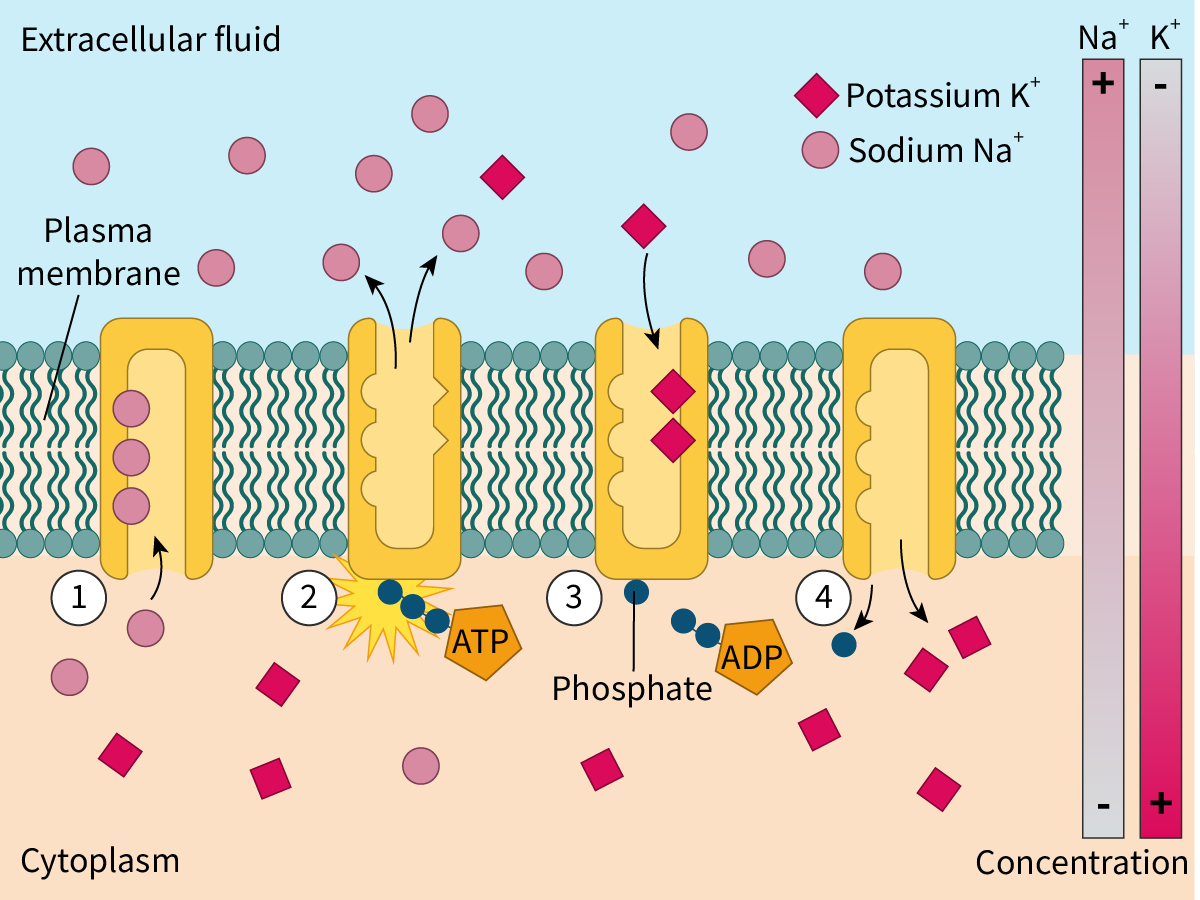
Figure 2. The sodium–potassium pump in action.
Thus, for every molecule of ATP hydrolysed, three sodium ions are pumped out and two potassium ions are pumped in. This builds a high concentration of K+ inside the cells. The Na+/K+ pump thus helps to establish and maintain the voltage across the membrane. It thereby plays an important role in re-establishing the membrane potential after the passage of a nerve impulse.
Indirect active transport
The Na+/K+ pump uses ATP as an energy source and is hence considered as direct active transport.
Indirect active transport is another mechanism. Here the source of energy is not ATP. The mechanism involves the transport of two solutes (ions or molecules); however, one solute is transported down its concentration gradient while the other is transported against its concentration gradient. The favourable movement (down the concentration gradient) is thereby coupled with an unfavourable movement (against the concentration gradient) and drives the latter.
Sodium ions bind to binding sites on the outer surface of the cotransporter.
Simultaneously, a molecule of glucose also binds to its binding site on the cotransporter.
This results in a conformational change that transports both the sodium ions and the glucose molecule to the inside of the cell.
The sodium gradient is maintained by the Na+/K+ pump which transports the sodium ions out of the cell.
Figure 3 illustrates this process.
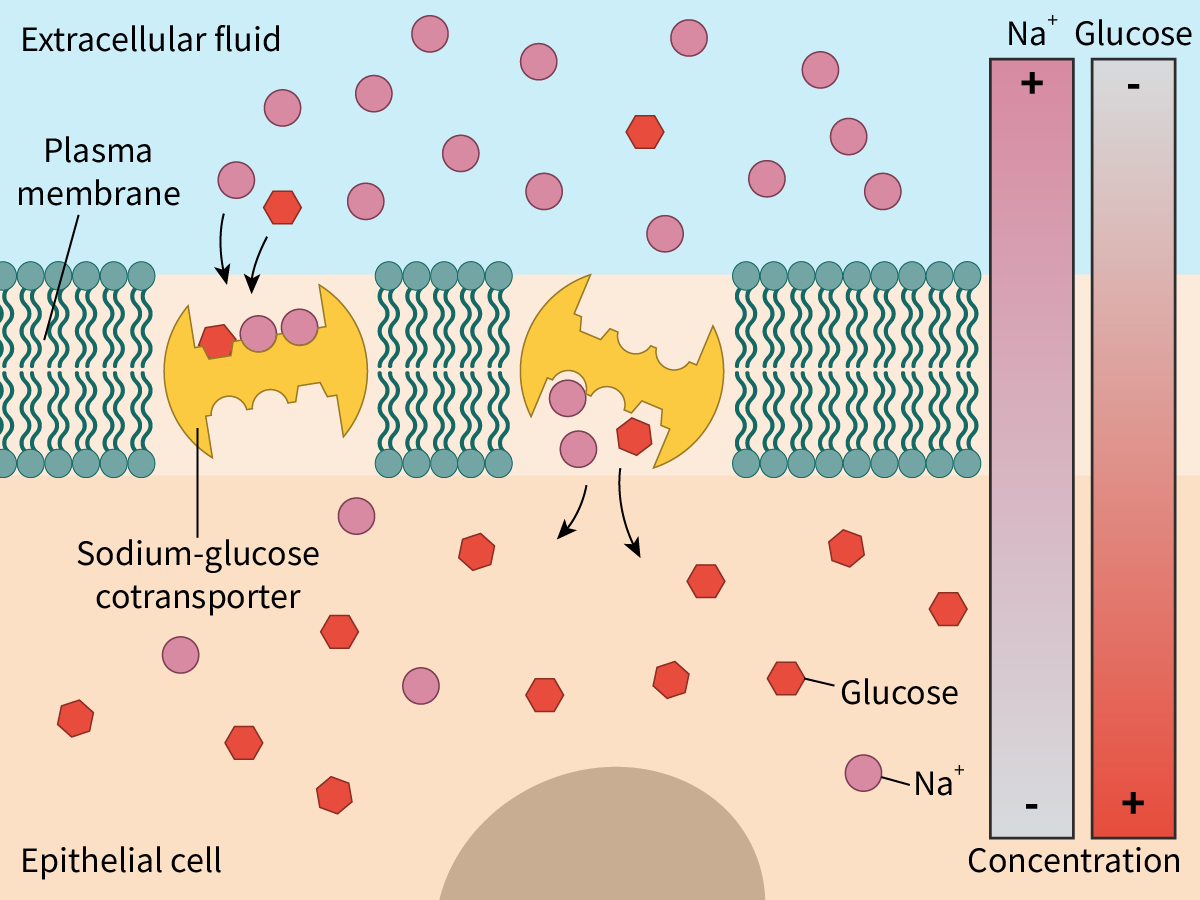
Figure 3. Indirect active transport.
Let us have a look at a couple of examples.
Soon after digestion, nutrient molecules like glucose or amino acids need to be transported from the intestinal lumen to the epithelial cells lining the small intestine, against their concentration gradient. This is an energy-requiring or
endergonic
process. The energy for this comes from the simultaneous transport of sodium ions, which is an energy-releasing or exergonic process as the ions are transported down their electrochemical gradient.
Indirect active transport also comes into play in the reabsorption of glucose by the cells of the nephron. Our kidneys filter blood. As the blood is filtered, along with the urea and waste material, large amounts of glucose and other useful substances are removed. The glucose molecules in the renal filtrate are reabsorbed (see section D3.3.7–8) with the help of the sodium-dependent glucose cotransporters present on the kidney epithelial cells, preventing the loss of glucose.
 Knowt
Knowt
