Stoichiometric Relationships
Unit 1: Particulate nature of matter
Elements
all substances are made up of one or more elements
over 100 known elements
the smallest part of an element is called an atom
Compounds
contains more than one element combined chemically in a fixed ratio
eg. water (two hydrogen atoms and one oxygen atom)
compounds have varying chemical and physical properties from their respective component elements
Mixtures
components may be elements or compounds
are not chemically bonded together
retain individual properties
all in the same stage = homogeneous
eg. air
States of Matter
Solid state
fixed shape and volume
particles held together by intermolecular forces
particles will vibrate at a fixed point but do not have translational velocity
turns into gaseous state by sublimation
turns into liquid state by melting
Liquid state
fixed volume
takes up the shape of the container
held together by intermolecular forces
turns into solid state by freezing
turns into gaseous state by boiling/vaporization/evaporation
Gaseous state
takes the space to completely fill container
widely spaced particles
intermolecular forces between particles are negligible
pressure is due to gaseous particles colliding with the walls of the container
turns into liquid state by condensation
turns into solid state by deposition
Unit 2: The mole concept and chemical formulas
Avogadro’s Constant
a single atom of an element has a small mass
this mass is known as Avogadro’s constant (NA)
the mass of one mole of any substance is known as the molar mass (M)
relative atomic mass (A): weighted mean of all the naturally occurring isotopes of the element
find the average
for molecules: relative molecular mass
for ionic compounds: relative formula mass
Formulas of Compounds
empirical formula: in simplest whole number the ratio of atoms of each element in a substance
obtained by knowing the mass of each element
molecular formula: shows the actual number of atoms in a substance
structural formula: shows the arrangement of atoms and bonds within a molecule
Unit 3: Chemical reactions and equations
Properties of Chemical Reactions
new substances are formed
bonds are broken and formed resulting in an energy change
fixed relationship between the number of particles and reactants
Chemical Equations
reactants are written on the left hand side
products are written on the right hand side
number of moles of each element must be the same on both sides in a balanced chemical equation'
single arrow shows reaction goes to completion
state symbols
s = solid
l = liquid
g = gas
aq = aqueous solution
coefficient refers to the number in front of the reactants and products in the equation
shows information on the molar ratio
Ionic Equations
ionic compounds completely dissociate in solution
spectator ions do not need to be written in the reaction
Unit 4: Mass and gaseous volume relationships
Measurement of molar quantities
solids: measure in mass (weighed)
liquids: weighed or volume recorded
density = mass/volume (units is g cm^-3)
gases: mass or volume
solutions: units is litre, dm³, cm³)
solute (dissolved substance) in is a known volume of solution (solute plus solvent)
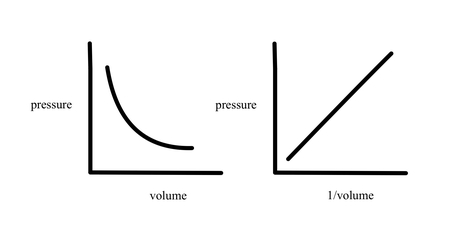
Fixed mass of gas
P = 1/ V
P = T
V = T
Ideal gas equation: PV = nRT
R = 8.31 (J K-1 mol-1)
Unit 5: Molar volume of a gas
ideal gas equation depends on the amount of gas but not on the nature of the gas
one mole of any gas will occupy the same volume at the same temperature and pressure
at 273K and 100kPa, the volume is 22.7 dm3
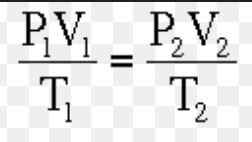
Calculations from equations
write down the correct formulas and all the reactants and products
balance the equation
work out the limiting reagent. the limiting reagent is the maximum yield to any of the products that can be determined
work out the amount (in mol) of the substance
convert to required unit
express solution in the correct number of significant digits and units