Hitting the Jackpot
Atoms
Subatomic particles
Atomic Number: Number of protons in the nucleus
Mass number: The number of nucleons
Dalton’s Atomic Theory
Isotope
Chemical properties-depend on the number of electrons, since elements have the same number of electrons, their chemical properties are identical
Physical properties: Depends on the neutrons of an atom, so the physical properties of isotopes pairs are slightly different’
Natural Abundance-Refers to the proportions of isotopes of a chemical elements as naturally found on a planet
Atomic mass is the relative percentage of all the naturally occuring isotopes of that element
Mass Spectra
How a mass spectrum for an element or molecule is created
A sample put into the spectrometer and vaporised;
An electron beam creates ions with a 1+ charge
Ions are accelerated by an electric field and focused
Ions are deflected by an electromagnet. Changing the strength of the electromagnetic changes the deflecting pattern based on ion mass
The ions are detected by the detector and the mass spectrum plotted
Emission spectra
Light: A form of electron magnetic radiations, a wave of it described with frequency and wavelength
Spectrum
A spectrum is produced when a light source (sunbeam, torch, laser etc) passes through a transparent prism (piece of glass, or a diffraction grating) and the light is dispersed through an angle that depends on the wavelength of the light passing through.
Continuous spectrum:
Contains all possible wavelengths (white light) then a continuous spectrum results
Line Spectra:
Only contains a few light colors surrounded by black. At higher frequencies they converge.

Emission spectrum of hydrogen
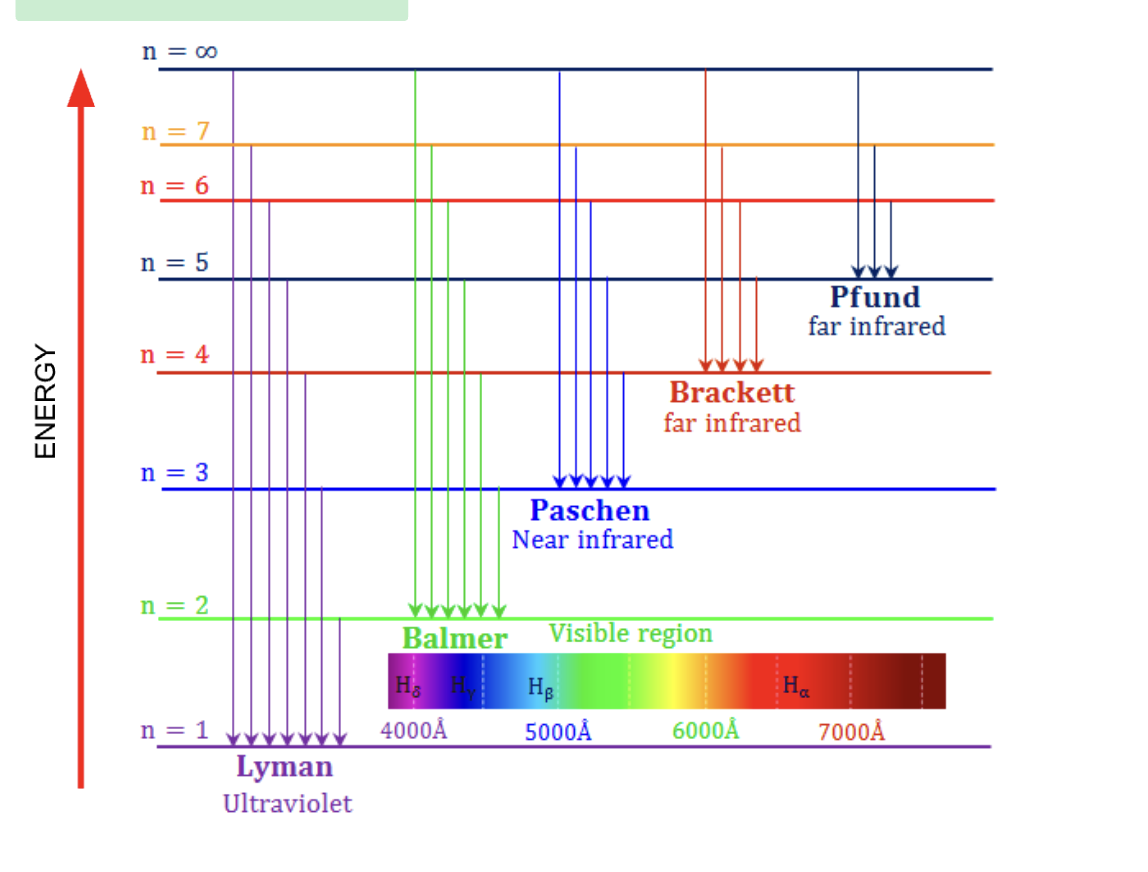
When electrons absorbs, heat or energy the go up energy levels, this is unstable so after some times they go down energy levels and emit photons at a specific wave length. Higher energy levels are closer together on the graph as they are closer on together in the atom, thus requiring less energy to move up and down. Each energy difference then refers to a specific wavelength. Use to prove the existence of energy levels
The energy of an electron in an energy level is equal to:
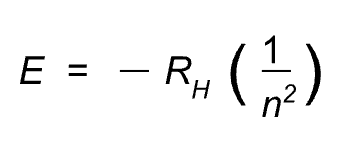
The energy emitted (or absorbed during energy level transition is equal to:
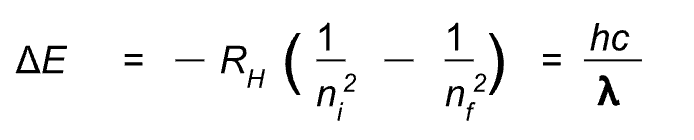
Quantum-Mechanical Model
Heisenberg uncertainty principle showed us that we cannot know both location and the velocity of very small particles such as the electron
Energy Levels and Orbitals
7 Energy levels, 7 periods of periodic table
S block:
First two groups of the periodic table
Spherical and only one orientation
So can only hold two electrons
P Block:
last 6 groups of periodic table (13-18)
Can hold 6 electrons as it can have three different directions (x, y and z)
D Block:
Transition metals
Contains 5 sub levels so maximum of 10 electrons
F Block:
Contains 7 atomic orbitals
Lanthanide and actinides
First energy levels can hold 2 electrons, then 8, 18, 32
Transition metals d block
Group 13-18 p block
Notable principles:
The Aufbau principle states that when adding electrons to an atom, the lower energy orbitals must be filled first. (s, p, d, f)
The Pauli exclusion principle states that an atomic orbital can only hold two electrons and they must have opposite spins.
Hund’s rule states that when we have degenerate orbitals (orbitals of the same energy) then each orbital is filled with a single electron before being doubly occupied turning them into a
4s sub level fills before 3d as it is lower in energy. However, 4s level electrons are taken away before 3d because 3d has lower energy.
Exceptions:
Every element in the period of Chromium and Copper have one less 4s electron but one more 3d electron. But then when leaving
Cu: 1s22s22p63s23p64s13d10
Cr [Ar] 4s1 3d5
Electron Configuration
Electron configuration is determined by distributing the atom’s electrons among the levels and sublevels based on a set of stated principles and a rule:
Aufbau Principle
An electron occupies the lowest energy orbital that can receive it. from s, p, d, f. This means that first 4s is filled as it is lower than 3d
Pauli Exclusion Principle
No two electrons in the same atom can have the same set of four quantum numbers.
Hund’s Rule
Orbitals of the same energy (degenerate orbitals) are occupied by one electron before any orbital is occupied by a second electron, and all electrons in separate singly occupied orbitals must have the same spin.
Exceptional Electron Configuration:
Chromium and copper have exceptions. Instead of having 4s2 one of the electrons is giving to d block so it can have half filled and fully filled blocked respectively
How to do it:
1 Fill up the electrons from the lowest energy level.
2 Put the electrons in each sub-energy level one at a time with spin up then pair up any more electrons added with spin down.
First IE Energy
Energy ionization energy is the energy required to remove one mole of the most loosely held electron from one mole gaseous atoms to produce to produce one mole gaseous ions each with an oxidation state +1
Equation for Ionisation energy:
M(g) → M+(g) + e–
IE is governed by:
the charge on the nucleus
the amount of screening by the inner electrons
the distance between the outer electrons and the nucleus
As you go down a group the increase in nuclear charge is the same as the increase in the number of inner electrons. However as you go down distance between nucleus and outer electrons increases so first ionization energy decreases.
IE goes up and down as removing an electron from a half filled or fully filled block is harder. But, overall there is an increase in IE as more neutrons creates stronger attractions pulling in electrons.
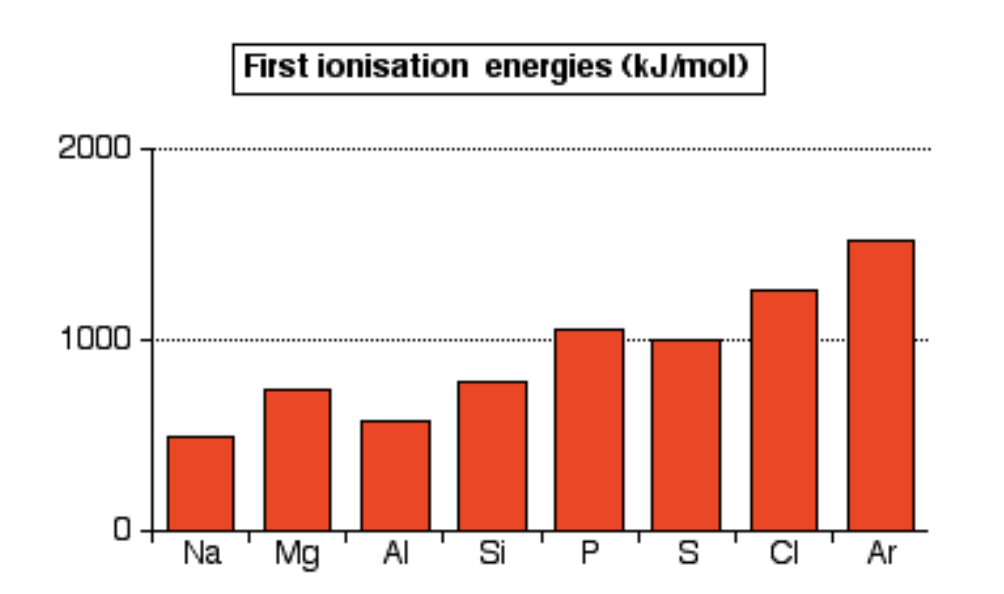
Electrons at higher energies are easier to remove as they are farther away from the nucleus
Ion formation
Ionic bonding
Ionic Compounds
Ionic compounds properties:
High melting and boiling points, due to high electrostatic attraction and crystal structure
Hard and Brittle as solids, due to hard electrostatic attraction however brittle as when hit positive and positive ions are repulsed overcoming electrostatic atraction
Non conductive when solid but conductive when liquid (molten or aqeous)
When dissolved in water called hydration
Lattice Enthalpy:
Definition: The energy required to convert one mole of solid ionic compound into gaseous ions.
Stability: Depends on all of the attraction between the actions and anions
The more energy released during the formation of the (solid), the more stable the solid is.
Strength depends on:
The charge on the ions, higher charge means, greater attraction between them and strong lattice enthalpy. More important than size
Size of the ions, smaller the atoms the stronger attraction between them and the stronger lattice energy
Metallic Bonding
A metal contains positive metal ions surrounded by a sea of delocalized electrons.
The smaller the cation and the more electrons in the ‘sea’ the stronger the metallic bonding and the higher the melting point
As you go down the group bonding strength decreases as atomic radii increases thus lower melting point
Across a period nuclear charges increases and atomic radii is constant so metallic bonding becomes strong so boiling point increases
Transition metals good at conducting electricity due to delocalized electrons
Malleability:
Be able to be compressed into shape without being broken
Ductility:
being able to be strecthced out
Transition elements
The periodic table
Periodicity
The atomic radius is half the distance between two nuclei of a diatomic molecule
Influenced by three factors:
Energy Level-Higher energy level is further away from nucleus
Charge on nucleus, more charge pulls electrons in closer
Shielding effect In a group, you add electrons for each level, this makes teh radii larger as you go down the table
Across a period
Number of protons increases so increase in nuclear charge
Electrons are filled in the same energy levels
So attraction increases thus atomic radii decreases
Down a group:
Number of energy levels increase
Protons increases, however the increase nuclear charge is exactly offset by electron shielding
Thus atomic radii increases
Ionic Radii:
The ionic radius is the radius of the most common ion formed
Positive ions:
When a cation is formed, an electron is removed, decreasing repulsion between electrons. Thus can be closer together, thus smaller radius
Negative ions:
when an anion is formed, an electron is gained. This increases the repulsion between electrons. The larger repulsion means a larger radius
Electronegativity:
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons
As the atoms get larger, any bonding pairs gets further and further away form the nucleus and so is less strongly attracted towards it. In other words, as you go down the group the elements become less electronegative.
Electron affinity:
Energy relased when an electron, in addition to the existing electrons, is attached to a neutral gaseous atom or. molecule. Similar trend to that it increases form left to right. Electron affinity decreases as you go down group 1 but the trend is not the same for all groups. If addition of an electron creates a less stable electron configuration the energy relased EA is less
Alloys
The periodic table
Periodic Law:
When elements are arranged in order of increasing atomic number their physical and chemical properties show a identifiable pattern
Group:
Also know as columns. Elements have similar characteristics. Is a series of elements that have the same valence electron number.
Period
The chemical properties gradually change from reactive metals ot reactive non metals
it is a sequence foofelements in which the same energy level is being filled
Is the number of energy levels that contains electrons.
Group 1 and 17 elements
Alkali Metals:
Reactivity increases down a group because the distance from teh nucleus to the valence electrons increases as you go down the group, the energy needed to remove that electron gets lower. This results in the reactiity increases as you go down a group.
Halogens:
Always diatomic molecules
Going down the group electron affinity decreases thus become more non metals and less reactive
Melting point of group 17:
As it goes down the group melting point increase because mass increase equals increase number of proton and electrons equals increase of IMF.
Oxides
Oxidation: Is when a species loses electrons
Reduction: Is when a species gains electrons
Acid Rain
Acid deposition refers to the acidic particles and liquids that deposit or fall to the Earth. It includes liquid deposition of acidic gases such as NOx and SO2 by rain, hail and snow solid deposition fo acidic gases and particles.
Rain is naturally acidic with a pH around 6. This is because of the high concentration of CO2 in the atmosphere.
Rule for determining oxidation state
1 | Free elements are assigned an oxidation state of 0 |
2 | The sum of the oxidation states of all the atoms in a species must be equal to the net charge on the species |
3 | The alkali metals in compounds are always assigned an oxidation state of +1 |
4 | Fluorine in compounds is always assigned na oxidation state of -1 |
5 | The alkaline earth metals and Zn and Cd in compounds are always assigned an oxidation state of 2+ |
6 | Hydrogen in compounds is assigned an oxidation state of -1 except in certain metal hydrides which is -1 |
7 | Oxygen in compounds is assigned an oxidation state of -2 except in peroxides H2O2 which is -1 |
8 | Halogen in compounds is assigned an oxidation state of -1 |
Charge vs Oxidation state: Charge is the loss or gain of electrons, oxidation state that number are assigned due to a set of rules allowing us to know if oxidation or reduction has happened.