
Chemistry - Unit 2: Matter & Energy
Topic 2.1 - Chemistry
Chemistry is the study of the composition, structure, and properties of matter.
Literal definition of chemistry: the branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.
We analyze the changes that matter undergoes and the energy associated with these changes.
The composition of matter refers to the way it’s made up. The structure of matter refers to how it’s combined and put together. The properties of matter define how it acts.
Topic 2.2 - Matter
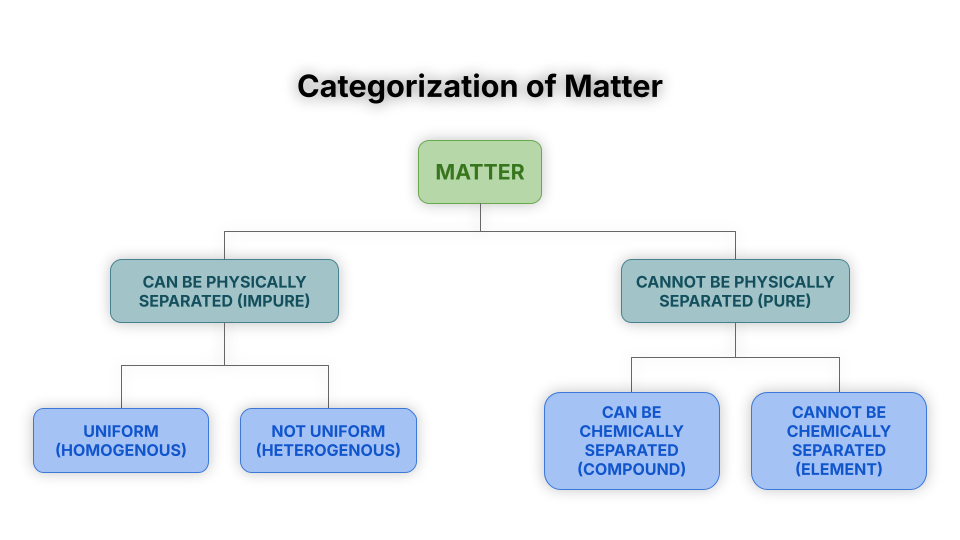
Matter is anything that has mass and takes up space.
Matter is placed into two categories:
Pure Matter / Pure Substances
Impure Matter / Mixtures
The diagram below shows how to categorize matter.
Topic 2.3 - Elements
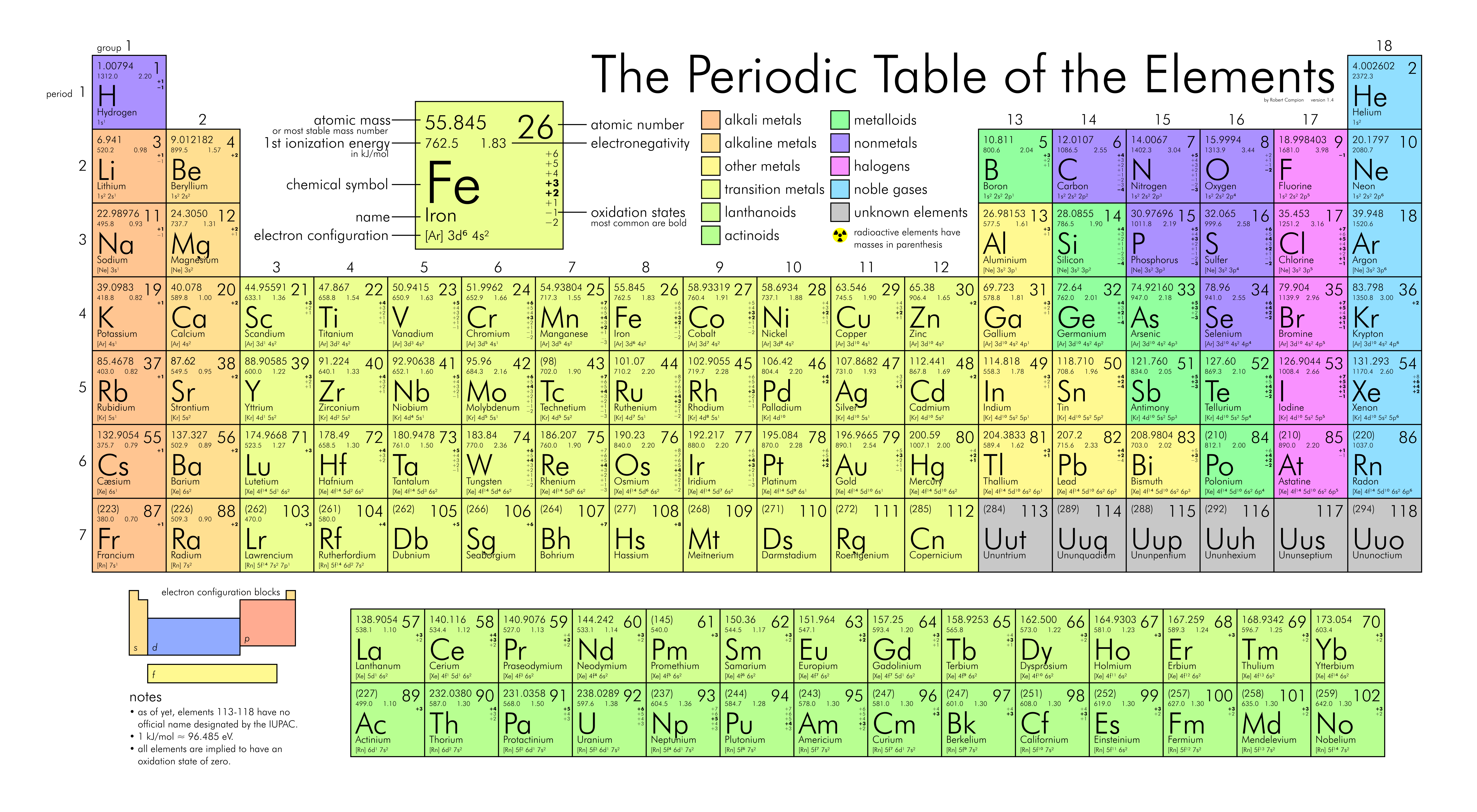
Elements are pure substances composed of identical atoms with the same atomic number (number of protons).
Elements cannot be physically or atomically broken down.
Elements are found on the periodic table of elements (shown below).
Topic 2.4 - Compounds
Compounds are combinations of two or more different elements that have been chemically combined (put together).
Properties and composition are definite in all samples of the compound.
Compounds can be decomposed by chemical means only.
Properties of the compound are different from those of its components.
Chemical formula for table salt: 2Na (s) + Cl2 (g) → 2NaCl (s)
Proportionality: In H2O (water), for every two hydrogen atoms there is one oxygen atom.
Topic 2.5 - Mixtures
Mixtures are composed of two or more different substances.
Substances in mixtures are physically combined.
Composition can vary through different samples.
Mixtures can only be broken down physically. Examples of this include boiling and filtering.
Mixtures always retain the properties of individual components.
Topic 2.5.1 - Homogenous Mixtures
Homogenous mixtures are uniformly and evenly mixed throughout.
Samples of homogenous mixtures will have the same composition
Aqueous solutions are homogenous water-based solutions. Examples include salt water and sugar water.
Topic 2.5.2 - Heterogeneous Mixtures
Heterogenous mixtures are not uniformly or evenly mixed throughout.
Samples of heterogeneous mixtures will have varying compositions.
Topic 2.6 - Physical & Chemical Properties
Most elements exist as monatomic elements (one atom). However, some elements exist as diatomic elements (two atoms).
There are seven diatomic elements. Use the mnemonic: “Have No Fear Of Ice Cold Beer.”
H2 (Hydrogen)
N2 (Nitrogen)
F2 (Fluorine)
O2 (Oxygen)
I2 (Iodine)
Cl2 (Chlorine)
Br2 (Bromine)
Topic 2.6.1 - Physical Properties
Physical properties are characteristics of a substance that can be observed or measured.
Some properties depend on size or amount, while others do not.
Differences in properties help separate one substance from another. Examples include density, melting point, boiling point, and atomic radius.
Topic 2.6.2 - Physical Changes
Physical changes are changes of a substance from one form to another without changing the chemical composition.
Water can change between a gas (vapor), a liquid, or a solid (ice).
Examples include:
Dissolution: NaCl (s) → Na+ (aq) + Cl- (aq)
Size changes: cutting up or ripping a piece of paper.
Topic 2.6.3 - Chemical Properties
Chemical properties are characteristics of a substance that is observed or measured through the interactions they have with other substances.
Remember the following key words:
Burning
Decomposing
Spoiling
Rusting/oxidizing
Reacting with
Combining with
Topic 2.6.4 - Chemical Changes
Chemical changes are changes in the composition (make up) and properties of one substance to those of other substances.
The result is a new substance with different properties.
Identity of the substance changes.
Examples include synthesis (2 reactants → 1 product), decomposition (1 product → 2 reactants), single replacement (A + BC → B + AC), and double replacement (AB + CD → CB + AD).
Topic 2.6.5 - Physical & Chemical Properties Vocabulary
Chemical Properties | Physical Properties |
Flammability | Color |
Decomposable | Texture |
Reactivity | Viscosity |
Combinable | Density |
Mass | |
Freezing/Melting/Boiling Point | |
Electrical Conductivity | |
Luster (Shine) | |
Hardness |
Topic 2.7 - Intrinsic vs. Extrinsic Properties
Intrinsic properties do not depend on the amount of matter in a sample.
Examples include temperature, boiling point, concentration, and luster.
Extrinsic properties depends on a sample’s mass.
Examples include weight, length, volume, and entropy (amount of clutter or disorder).
Topic 2.8 - Separation of Mixtures
Mixtures are combined by physical processes.
Each substance in a mixture has different chemical and physical properties.
There are different separation techniques.
Properties leading to separation include density, molecular polarity (when one side of the molecule is more positive or negative than the other), freezing/boiling point, and magnetism.
Topic 2.8.1 - Separation with a Magnet
Using a magnet to draw out a metal.
For example, in a mixture of iron and sand, iron retains its magnetism. You can use a magnet to draw the iron out of the heterogeneous mixture.
Topic 2.8.2 - Filtration
Filtration refers to separation by particle size.
Separates a solid from a liquid.
The liquid passes through the filter paper, leaving the solid trapped in the paper.
Topic 2.8.3 - Watch Glass / Crucible Evaporation
Separation based on boiling points.
Mixture is heated up to boil off each substance.
Goes in order from lowest to highest boiling point.
Topic 2.8.4 - Distillation
Process by which liquids or liquids and solids can be separated by their boiling points.
Examples include extracting gasoline from petroleum or separating salt water into salt and water.
Topic 2.8.5 - Chromatography
The mixture is dissolved in a solvent (mobile phase).
Components move through a stationary phase at different speeds.
The mixture is separated based on solubility and size.
Topic 2.8.6 - Centrifuge
The mixture is separated based on density.
The heavier substance falls to the bottom, while the lighter substance floats at the top.
Topic 2.9 - Energy
Energy is the capacity for doing work.
Physical and chemical changes result in changes in energy.
Work is a force that causes an object to move or change its existing motion.
There are two forms of energy: mechanical and non-mechanical energy.
Topic 2.9.1 - Mechanical Energy
There are two components to mechanical energy: potential (stored) energy and kinetic energy (motion).
Kinetic and potential energy explain many physical processes.
Topic 2.9.2 - Non-Mechanical Energy
Chemical energy powers chemical reactions.
Light energy powers light waves.
Electrical energy powers electrical currents.
Atomic/nuclear energy is associated with the changes in the nucleus of an atom.
Topic 2.9.3 - Thermal (Heat) Energy
Thermal energy is associated with the random motion of atoms and molecules and the mass.
Heat transfers from a body of higher temperature to a body of lower temperature until they are both the same temperature (equilibrium). (The second law of thermodynamics / the rule of heat flow.)
Topic 2.9.4 - Law of Conservation of Energy
Energy cannot be created or destroyed, but may be transformed from one form to another.
The total energy of an isolated system remains constant.
This law applies to closed systems, where energy can only change through entering or leaving the system.
Topic 2.9.5 - Heat and Temperature
Heat is a type of energy.
Energy is measured in joules.
Temperature is a measure of the average level of molecular motion (the average kinetic energy of molecules).
A higher temperature means more kinetic energy, while a lower temperature means less kinetic energy.
Topic 2.10 - The Phases of Matter
The three known phases of matter are solid, liquid, and gas.
Each phase has its own properties and amount of molecular motion
Solid | Liquid | Gas | |
Shape | Definite shape. | Takes shape of container. | No definite shape. |
Volume | Definite volume. | Definite volume. | No definite volume. |
Motion | Particles vibrate in place. | Particles slide past each other. | Molecules are distant and move in straight lines in all directions. |
Topic 2.10.1 - Phase Changes
Phase changes occur at different temperatures and pressures.
Every substance has its own unique melting and boiling points.
Phase changes are physical changes only. Substances change state without changing its chemical composition.
Most substances require a large change in temperature or pressure to change phase.
Topic 2.10.2 - Types of Reactions for Phase Changes
Energy is released or absorbed during a physical or chemical change.
There are two types of reactions:
During exothermic reactions, energy is released; the reaction will feel hot.
Chemical energy becomes heat energy.
* Any cooling process. (Deposition, condensation, or freezing.)
During endothermic reactions, energy is absorbed; the reaction will feel cold.
Heat energy becomes chemical energy.
* Any heating process. (Sublimation, evaporation, or melting.)
The kinetic energy either increases or decreases. The addition of heat causes molecules to spread out.

Unlike heating, cooling brings the molecules together.
At any phase change, the two phases are existing at the same time. The force of attraction can increase (while cooling) or decrease (while heating).
Solids are generally the most ordered.

Topic 2.10.3 - Phase Changes Vocabulary
The melting point is the temperature at which a substance begins to melt.
The freezing point is the temperature at which the force of attraction increases between the molecules (a transition from solid to liquid).
The boiling point is the temperature at which the force of attraction decreases (a transition from liquid to gas).
Topic 2.11 - Heat of Fusion, Heat of Vaporization, & Specific Heat Capacity
Heat of fusion is the amount of energy needed to convert some mass of a substance from solid to liquid (or vice versa).
Heat of vaporization is the amount of energy needed to convert some mass of a substance from liquid to gas (or vice versa).
Specific heat capacity is the amount of energy needed to increase the temperature of a substance by one unit (degrees Celsius or Kelvin).
Topic 2.11.1 - The Three Heat Equations
q = mHf (phase change) | q = mHv (phase change) | q = mCΔT (in phase) |
q = amount of heat (J) | q = amount of heat (J) | q = amount of heat (J) |
m = mass (g) | m = mass (g) | m = mass (g) |
Hf = heat of fusion (s → l or l → s) | Hv = heat of vaporization (l → g or g → l) | C = specific heat capacity |
ΔT = change in temperature |

Topic 2.11.2 - Solving the Heat Equations:
Identify whether you are in phase or undergoing a phase change.
Look for “melting” or “boiling” when talking about a phase change.
Correctly select which equation to use.
q = mHf (when undergoing a phase change)
q = mHv (when undergoing a phase change)
q = mCΔT (when in phase)
Substitute your values into the equations, then solve for q.
Chemistry - Unit 2: Matter & Energy
Topic 2.1 - Chemistry
Chemistry is the study of the composition, structure, and properties of matter.
Literal definition of chemistry: the branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.
We analyze the changes that matter undergoes and the energy associated with these changes.
The composition of matter refers to the way it’s made up. The structure of matter refers to how it’s combined and put together. The properties of matter define how it acts.
Topic 2.2 - Matter
Matter is anything that has mass and takes up space.
Matter is placed into two categories:
Pure Matter / Pure Substances
Impure Matter / Mixtures
The diagram below shows how to categorize matter.

Topic 2.3 - Elements
Elements are pure substances composed of identical atoms with the same atomic number (number of protons).
Elements cannot be physically or atomically broken down.
Elements are found on the periodic table of elements (shown below).

Topic 2.4 - Compounds
Compounds are combinations of two or more different elements that have been chemically combined (put together).
Properties and composition are definite in all samples of the compound.
Compounds can be decomposed by chemical means only.
Properties of the compound are different from those of its components.
Chemical formula for table salt: 2Na (s) + Cl2 (g) → 2NaCl (s)
Proportionality: In H2O (water), for every two hydrogen atoms there is one oxygen atom.
Topic 2.5 - Mixtures
Mixtures are composed of two or more different substances.
Substances in mixtures are physically combined.
Composition can vary through different samples.
Mixtures can only be broken down physically. Examples of this include boiling and filtering.
Mixtures always retain the properties of individual components.
Topic 2.5.1 - Homogenous Mixtures
Homogenous mixtures are uniformly and evenly mixed throughout.
Samples of homogenous mixtures will have the same composition
Aqueous solutions are homogenous water-based solutions. Examples include salt water and sugar water.
Topic 2.5.2 - Heterogeneous Mixtures
Heterogenous mixtures are not uniformly or evenly mixed throughout.
Samples of heterogeneous mixtures will have varying compositions.
Topic 2.6 - Physical & Chemical Properties
Most elements exist as monatomic elements (one atom). However, some elements exist as diatomic elements (two atoms).
There are seven diatomic elements. Use the mnemonic: “Have No Fear Of Ice Cold Beer.”
H2 (Hydrogen)
N2 (Nitrogen)
F2 (Fluorine)
O2 (Oxygen)
I2 (Iodine)
Cl2 (Chlorine)
Br2 (Bromine)
Topic 2.6.1 - Physical Properties
Physical properties are characteristics of a substance that can be observed or measured.
Some properties depend on size or amount, while others do not.
Differences in properties help separate one substance from another. Examples include density, melting point, boiling point, and atomic radius.
Topic 2.6.2 - Physical Changes
Physical changes are changes of a substance from one form to another without changing the chemical composition.
Water can change between a gas (vapor), a liquid, or a solid (ice).
Examples include:
Dissolution: NaCl (s) → Na+ (aq) + Cl- (aq)
Size changes: cutting up or ripping a piece of paper.
Topic 2.6.3 - Chemical Properties
Chemical properties are characteristics of a substance that is observed or measured through the interactions they have with other substances.
Remember the following key words:
Burning
Decomposing
Spoiling
Rusting/oxidizing
Reacting with
Combining with
Topic 2.6.4 - Chemical Changes
Chemical changes are changes in the composition (make up) and properties of one substance to those of other substances.
The result is a new substance with different properties.
Identity of the substance changes.
Examples include synthesis (2 reactants → 1 product), decomposition (1 product → 2 reactants), single replacement (A + BC → B + AC), and double replacement (AB + CD → CB + AD).
Topic 2.6.5 - Physical & Chemical Properties Vocabulary
Chemical Properties | Physical Properties |
Flammability | Color |
Decomposable | Texture |
Reactivity | Viscosity |
Combinable | Density |
Mass | |
Freezing/Melting/Boiling Point | |
Electrical Conductivity | |
Luster (Shine) | |
Hardness |
Topic 2.7 - Intrinsic vs. Extrinsic Properties
Intrinsic properties do not depend on the amount of matter in a sample.
Examples include temperature, boiling point, concentration, and luster.
Extrinsic properties depends on a sample’s mass.
Examples include weight, length, volume, and entropy (amount of clutter or disorder).
Topic 2.8 - Separation of Mixtures
Mixtures are combined by physical processes.
Each substance in a mixture has different chemical and physical properties.
There are different separation techniques.
Properties leading to separation include density, molecular polarity (when one side of the molecule is more positive or negative than the other), freezing/boiling point, and magnetism.
Topic 2.8.1 - Separation with a Magnet
Using a magnet to draw out a metal.
For example, in a mixture of iron and sand, iron retains its magnetism. You can use a magnet to draw the iron out of the heterogeneous mixture.
Topic 2.8.2 - Filtration
Filtration refers to separation by particle size.
Separates a solid from a liquid.
The liquid passes through the filter paper, leaving the solid trapped in the paper.
Topic 2.8.3 - Watch Glass / Crucible Evaporation
Separation based on boiling points.
Mixture is heated up to boil off each substance.
Goes in order from lowest to highest boiling point.
Topic 2.8.4 - Distillation
Process by which liquids or liquids and solids can be separated by their boiling points.
Examples include extracting gasoline from petroleum or separating salt water into salt and water.
Topic 2.8.5 - Chromatography
The mixture is dissolved in a solvent (mobile phase).
Components move through a stationary phase at different speeds.
The mixture is separated based on solubility and size.
Topic 2.8.6 - Centrifuge
The mixture is separated based on density.
The heavier substance falls to the bottom, while the lighter substance floats at the top.
Topic 2.9 - Energy
Energy is the capacity for doing work.
Physical and chemical changes result in changes in energy.
Work is a force that causes an object to move or change its existing motion.
There are two forms of energy: mechanical and non-mechanical energy.
Topic 2.9.1 - Mechanical Energy
There are two components to mechanical energy: potential (stored) energy and kinetic energy (motion).
Kinetic and potential energy explain many physical processes.
Topic 2.9.2 - Non-Mechanical Energy
Chemical energy powers chemical reactions.
Light energy powers light waves.
Electrical energy powers electrical currents.
Atomic/nuclear energy is associated with the changes in the nucleus of an atom.
Topic 2.9.3 - Thermal (Heat) Energy
Thermal energy is associated with the random motion of atoms and molecules and the mass.
Heat transfers from a body of higher temperature to a body of lower temperature until they are both the same temperature (equilibrium). (The second law of thermodynamics / the rule of heat flow.)
Topic 2.9.4 - Law of Conservation of Energy
Energy cannot be created or destroyed, but may be transformed from one form to another.
The total energy of an isolated system remains constant.
This law applies to closed systems, where energy can only change through entering or leaving the system.
Topic 2.9.5 - Heat and Temperature
Heat is a type of energy.
Energy is measured in joules.
Temperature is a measure of the average level of molecular motion (the average kinetic energy of molecules).
A higher temperature means more kinetic energy, while a lower temperature means less kinetic energy.
Topic 2.10 - The Phases of Matter
The three known phases of matter are solid, liquid, and gas.
Each phase has its own properties and amount of molecular motion
Solid | Liquid | Gas | |
Shape | Definite shape. | Takes shape of container. | No definite shape. |
Volume | Definite volume. | Definite volume. | No definite volume. |
Motion | Particles vibrate in place. | Particles slide past each other. | Molecules are distant and move in straight lines in all directions. |
Topic 2.10.1 - Phase Changes
Phase changes occur at different temperatures and pressures.
Every substance has its own unique melting and boiling points.
Phase changes are physical changes only. Substances change state without changing its chemical composition.
Most substances require a large change in temperature or pressure to change phase.
Topic 2.10.2 - Types of Reactions for Phase Changes
Energy is released or absorbed during a physical or chemical change.
There are two types of reactions:
During exothermic reactions, energy is released; the reaction will feel hot.
Chemical energy becomes heat energy.
* Any cooling process. (Deposition, condensation, or freezing.)
During endothermic reactions, energy is absorbed; the reaction will feel cold.
Heat energy becomes chemical energy.
* Any heating process. (Sublimation, evaporation, or melting.)
The kinetic energy either increases or decreases. The addition of heat causes molecules to spread out.

Unlike heating, cooling brings the molecules together.
At any phase change, the two phases are existing at the same time. The force of attraction can increase (while cooling) or decrease (while heating).
Solids are generally the most ordered.

Topic 2.10.3 - Phase Changes Vocabulary
The melting point is the temperature at which a substance begins to melt.
The freezing point is the temperature at which the force of attraction increases between the molecules (a transition from solid to liquid).
The boiling point is the temperature at which the force of attraction decreases (a transition from liquid to gas).
Topic 2.11 - Heat of Fusion, Heat of Vaporization, & Specific Heat Capacity
Heat of fusion is the amount of energy needed to convert some mass of a substance from solid to liquid (or vice versa).
Heat of vaporization is the amount of energy needed to convert some mass of a substance from liquid to gas (or vice versa).
Specific heat capacity is the amount of energy needed to increase the temperature of a substance by one unit (degrees Celsius or Kelvin).
Topic 2.11.1 - The Three Heat Equations
q = mHf (phase change) | q = mHv (phase change) | q = mCΔT (in phase) |
q = amount of heat (J) | q = amount of heat (J) | q = amount of heat (J) |
m = mass (g) | m = mass (g) | m = mass (g) |
Hf = heat of fusion (s → l or l → s) | Hv = heat of vaporization (l → g or g → l) | C = specific heat capacity |
ΔT = change in temperature |

Topic 2.11.2 - Solving the Heat Equations:
Identify whether you are in phase or undergoing a phase change.
Look for “melting” or “boiling” when talking about a phase change.
Correctly select which equation to use.
q = mHf (when undergoing a phase change)
q = mHv (when undergoing a phase change)
q = mCΔT (when in phase)
Substitute your values into the equations, then solve for q.
 Knowt
Knowt