WK4: Care of the Fetus
EMBRYONIC STRUCTURE
A. The Decidua
After fertilization, the corpus luteum in the ovary continues to function rather than atrophying, because of the influence of human chorionic gonadotropin (hCG), a hormone secreted by the trophoblast cells. This causes the uterine endometrium to continue to grow in thickness and vascularity, instead of sloughing off as in a usual menstrual cycle. The endometrium is now termed the decidua (the Latin word for “falling off”), because it will be discarded after the birth of the child.
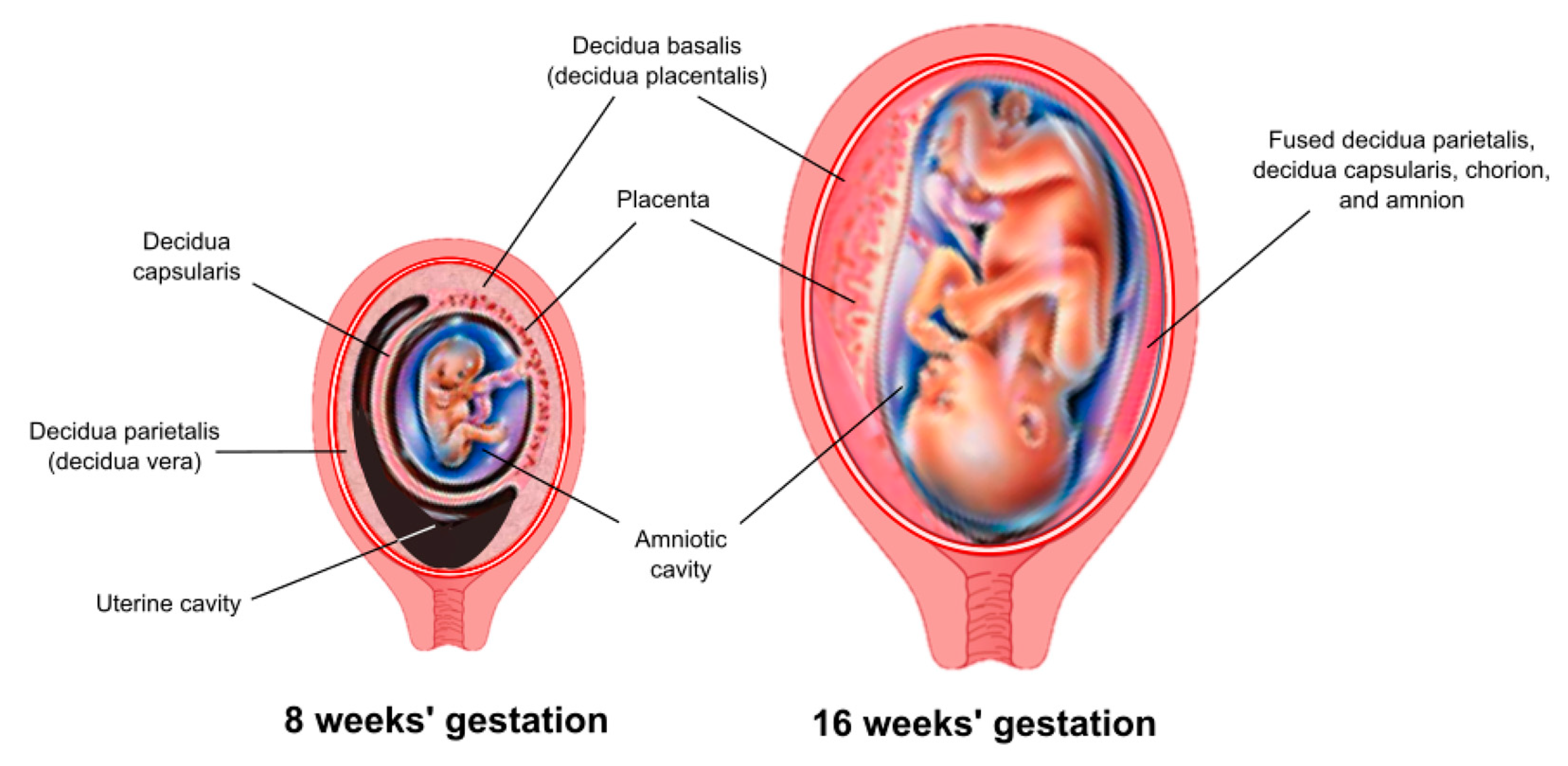
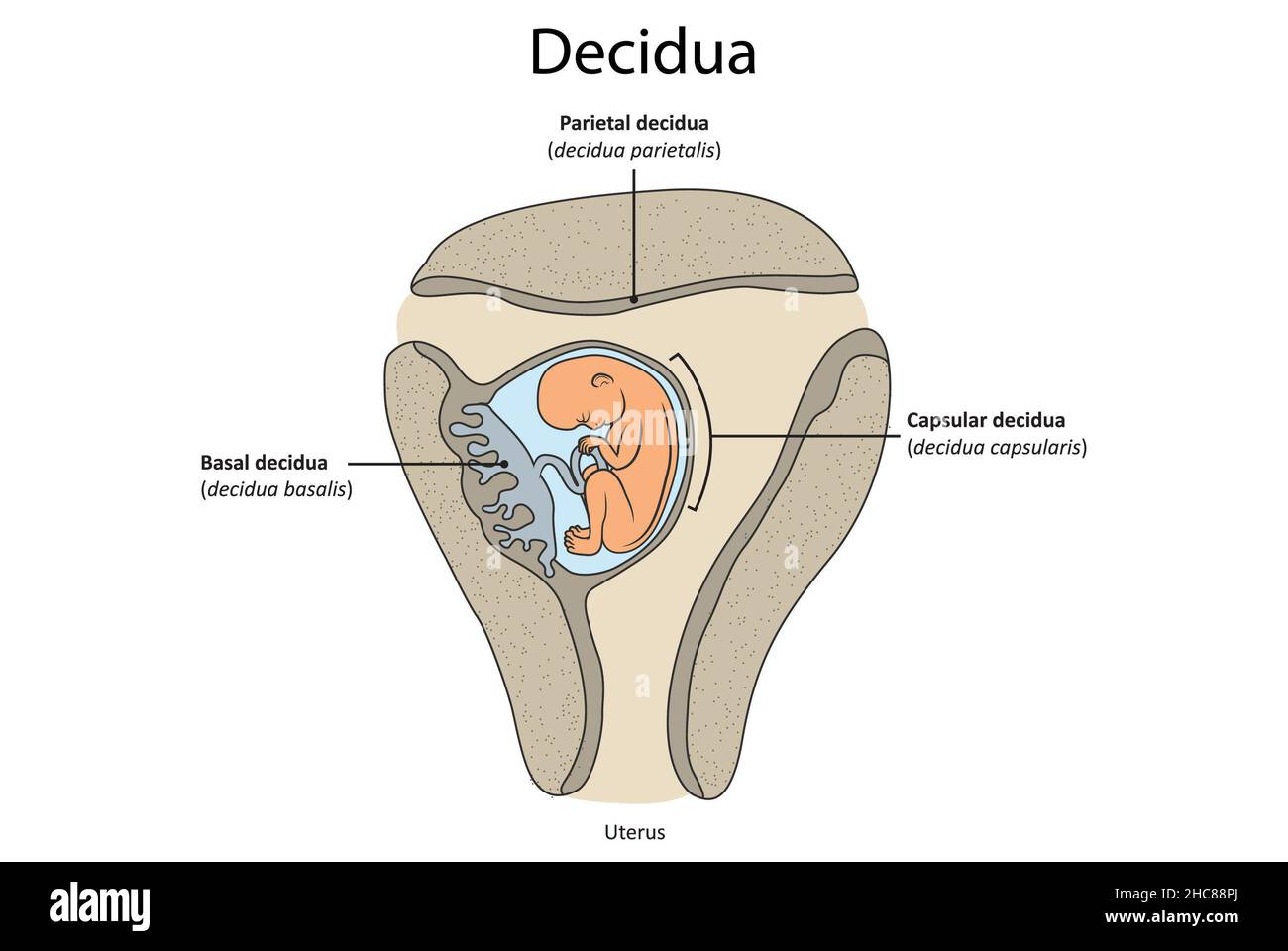
The decidua has three separate areas:
Decidua basalis, the part of the endometrium that lies directly under the embryo (or the portion where the trophoblast cells establish communication with maternal blood vessels)
Decidua capsularis, the portion of the endometrium that stretches or encapsulates the surface of the trophoblast
Decidua vera/parietalis, the remaining portion of the uterine lining. As the embryo continues to grow, it pushes the decidua capsularis before it like a blanket. Eventually, the embryo enlarges so much that this action brings the decidua capsularis into contact with the opposite uterine wall (the decidua vera). Here, the two decidua areas fuse, which is why, at birth, the entire inner surface of the uterus is stripped away, leaving the organ highly susceptible to hemorrhage and infection
B. Chorionic Villi
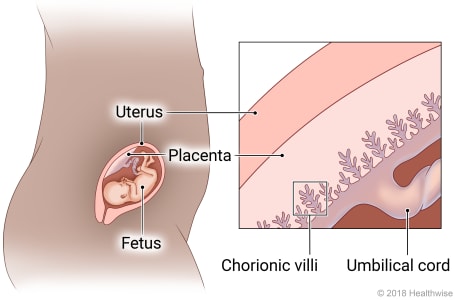
Once implantation is complete, the trophoblastic layer of cells of the blastocyst begins to mature rapidly. As early as the 11th or 12th day, miniature villi that resemble probing fingers, termed chorionic villi, reach out from the single layer of cells into the uterine endometrium to begin the formation of the placenta. At term, almost 200 such villi will have formed (Knuppel, 2007).
All chorionic villi have a central core of connective tissue and fetal capillaries. A double layer of trophoblast cells surrounds these. The outer of the two covering layers is the syncytiotrophoblast or the syncytial layer (outer and inner). This layer of cells produces various placental hormones, such as hCG, somatomammotropin (human placental lactogen [hPL]), estrogen, and progesterone.
The middle layer, the cytotrophoblast or Langhans’ layer, is present as early as 12 days’ gestation. It appears to function early in pregnancy to protect the growing embryo and fetus from certain infectious organisms such as the spirochete of syphilis. This layer of cells disappears, however, between the 20th and 24th weeks. This is why syphilis is not considered to have a high potential for fetal damage early in pregnancy, only after the point at which cytotrophoblast cells are no longer present (Ainbinder, Ramin, & DeCherney, 2007). The layer appears to offer little protection against viral invasion at any point



The Placenta
The placenta (Latin for “pancake,” which is descriptive of its size and appearance at term) arises out of the continuing growth of trophoblast tissue. Its growth parallels that of the fetus, growing from a few identifiable cells at the beginning of pregnancy to an organ 15 to 20 cm in diameter and 2 to 3 cm in-depth, covering about half the surface area of the internal uterus at term.
Circulation
As early as the 12th day of pregnancy, maternal blood begins to collect in the intervillous spaces of the uterine endometrium surrounding the chorionic villi. By the third week, oxygen and other nutrients, such as glucose, amino acids, fatty acids, minerals, vitamins, and water, osmose from the maternal blood through the cell layers of the chorionic villi into the villi capillaries. From there, nutrients are transported to the developing embryo.
Placental osmosis is so effective that all except a few substances can cross from the mother into the fetus. Because almost all drugs can cross into the fetal circulation, a woman must take no nonessential drugs (including alcohol and nicotine) during pregnancy (Rogers-Adkinson & Stuart, 2007).
For practical purposes, because the process of osmosis is so effective, there is no direct exchange of blood between the embryo and the mother during pregnancy. Because the outer chorionic villi layer is only one cell thick after the third trimester minute breaks do occur and allow occasional fetal cells to cross into the maternal bloodstream, as well as fetal enzymes such as alpha-fetoprotein (AFP) from the fetal liver.
About 100 maternal uterine arteries supply the mature placenta. To provide enough blood for exchange, the rate of uteroplacental blood flow in pregnancy increases from about 50 mL/min at 10 weeks to 500 to 600 mL/min at term. The woman’s heart rate, total cardiac output, and blood volume increase to supply blood to the placenta
Uterine perfusion, and thus placental circulation, is most efficient when the woman lies on her left side. This position lifts the uterus away from the inferior vena cava, preventing blood from being trapped in the woman’s lower extremities. If the woman lies on her back and the weight of the uterus compresses the vena cava, placental circulation can be so sharply reduced that supine hypotension (very low maternal blood pressure and poor uterine circulation) occurs (Knuppel, 2007).
At term, the placental circulatory network has grown so extensively that a placenta weighs 400 to 600 g (1 lb), one-sixth the weight of the baby. If a placenta is smaller than this, it suggests that circulation to the fetus may have been inadequate. A placenta larger than this may also indicate that circulation to the fetus was threatened because it suggests that the placenta was forced to spread out unusually to maintain a sufficient blood supply. The fetus of a woman with diabetes may also develop a larger-than-usual placenta from excess fluid collected between cells.
Endocrine Function
Aside from serving as the conduit for oxygen and nutrients for the fetus, the syncytial (outer) layer of the chorionic villi develops into a separate, important hormone-producing system.
1. Human Chorionic Gonadotropin.
The first placental hormone produced, hCG, can be found in maternal blood and urine as early as the first missed menstrual period (shortly after implantation has occurred) through about the 100th day of pregnancy. Because this is the hormone analyzed by pregnancy tests, a false-negative result from a pregnancy test may be obtained before or after this period. The woman’s blood serum will be completely negative for HCG within 1 to 2 weeks after birth. Testing for hCG after birth can be used as proof that placental tissue is no longer present.
The purpose of hCG is to act as a fail-safe measure to ensure that the corpus luteum of the ovary continues to produce progesterone and estrogen. This is important because if the corpus luteum should fail and the level of progesterone falls, the endometrial lining will slough and the pregnancy will be lost. hCG also may play a role in suppressing the maternal immunologic response so that placental tissue is not detected and rejected as a foreign substance.
2. Estrogen.
Estrogen (primarily estriol) is produced as a second product of the syncytial cells of the placenta. Estrogen contributes to the woman’s mammary gland development in preparation for lactation and stimulates uterine growth to accommodate the developing fetus.
3. Progesterone.
Estrogen is often referred to as the “hormone of women”; progesterone as the “hormone of mothers.” This is because, although estrogen influences a female appearance, progesterone is necessary to maintain the endometrial lining of the uterus during pregnancy. It is present in serum as early as the fourth week of pregnancy, as a result of the continuation of the corpus luteum. After placental synthesis begins (at about the 12th week), the level of progesterone rises progressively during the remainder of the pregnancy. This hormone also appears to reduce the contractility of the uterus during pregnancy, preventing premature labor.
4. Human Placental Lactogen (Human Chorionic Somatomammotropin)
hPL is a hormone with both growth-promoting and lactogenic (milk-producing) properties. It is produced by the placenta beginning as early as the sixth week of pregnancy, increasing to a peak level at term. It can be assayed in both maternal serum and urine. It promotes mammary gland (breast) growth in preparation for lactation in the mother. It also serves the important role of regulating maternal glucose, protein, and fat levels so that adequate amounts of these nutrients are always available to the fetus (Taylor & Lebovic, 2007).
Placental Proteins
The placenta also produces several plasma proteins. The function of these has not been well documented, but it is thought that they may contribute to decreasing the immunologic impact of the growing placenta through being part of the complement cascade (Knuppel, 2007).
C. The Amniotic Membranes
The chorionic villi on the medial surface of the trophoblast (those that are not involved in implantation, because they do not touch the endometrium) gradually thin, leaving the medial surface of the structure smooth (the chorion laeve, or smooth chorion). The smooth chorion eventually becomes the chorionic membrane, the outermost fetal membrane. Its purpose is to form the sac that contains the amniotic fluid. A second membrane lining the chorionic membrane, the amniotic membrane or amnion, forms beneath the chorion. Early in pregnancy, these membranes become so adherent that they seem as one at term. At birth they can be seen covering the fetal surface of the placenta, giving that surface its typically shiny appearance. There is no nerve supply, so when they spontaneously rupture at term or are artificially ruptured, neither woman nor child experiences any pain.
In contrast to the chorionic membrane, the amniotic membrane not only offers support to amniotic fluid but also actually produces the fluid. In addition, it produces a phospholipid that initiates the formation of prostaglandins, which can cause uterine contractions and maybe the trigger that initiates labor
D. The Amniotic Fluid
Amniotic fluid is constantly being newly formed and reabsorbed by the amniotic membrane, so it never becomes stagnant. Some of it is absorbed by direct contact with the fetal surface of the placenta. The major method of absorption, however, occurs because the fetus continually swallows the fluid. In the fetal intestine, it is absorbed into the fetal bloodstream. From there, it goes to the umbilical arteries and the placenta, and it is exchanged across the placenta. At term, the amount of amniotic fluid has increased so much that it ranges from 800 to 1200 mL
If for any reason the fetus is unable to swallow (esophageal atresia or anencephaly are the two most common reasons), excessive amniotic fluid, or hydramnios (more than 2000 mL in total, or pockets of fluid larger than 8 cm on ultrasound), will result. Hydramnios also tends to occur in women with diabetes, because hyperglycemia causes excessive fluid shifts into the amniotic space (Bush & Pernoll, 2007).
Early in fetal life, as soon as the fetal kidneys become active, fetal urine adds to the quantity of the amniotic fluid. A disturbance of kidney function may cause oligohydramnios, or a reduction in the amount of amniotic fluid (less than 300 mL in total, or no pocket on ultrasound larger than 1 cm) (Knuppel, 2007).
The most important purpose of amniotic fluid is to shield the fetus against pressure or a blow to the mother’s abdomen. Because liquid changes temperature more slowly than air, it also protects the fetus from temperature changes. As yet another function, it aids in muscular development, because it allows the fetus freedom to move. Finally, it protects the umbilical cord from pressure, protecting the fetal oxygen supply. Even if the amniotic membranes rupture before birth and the bulk of amniotic fluid is lost, some will always surround the fetus in utero, because new fluid is constantly formed. Amniotic fluid is slightly alkaline, with a pH of about 7.2. Checking the pH of the fluid at the time of rupture helps to differentiate it from urine, which is acidic (pH 5.0–5.5).
E. The Umbilical Cord
The umbilical cord is formed from the fetal membranes (amnion and chorion) and provides a circulatory pathway that connects the embryo to the chorionic villi of the placenta. Its function is to transport oxygen and nutrients to the fetus from the placenta and to return waste products from the fetus to the placenta. It is about 53 cm (21 in) in length at term and about 2 cm (3 ⁄4 in) thick. The bulk of the cord is a gelatinous mucopolysaccharide called Wharton’s jelly, which gives the cord body and prevents pressure on the vein and arteries that pass through it. The outer surface is covered with amniotic membrane.
An umbilical cord contains only one vein (carrying blood from the placental villi to the fetus) but two arteries (carrying blood from the fetus back to the placental villi). The number of veins and arteries in the cord is always assessed and recorded at birth because about 1% to 5% of infants are born with a cord that contains only a single vein and artery. From 15% to 20% of these infants are found to have accompanying chromosomal disorders or congenital anomalies, particularly of the kidney and heart (Lubusky et al., 2007). Blood can be withdrawn from the umbilical vein or transfused into the vein during intrauterine life for fetal assessment or treatment (termed percutaneous umbilical blood sampling [PUBS]).
In about 20% of all births, a loose loop of cord is found around the fetal neck (nuchal cord) at birth. If this loop of cord is removed before the newborn’s shoulders are born, so that there is no traction on it, the oxygen supply to the fetus remains unimpaired (Jackson, Melvin, & Downe, 2007). Because the umbilical cord contains no nerve supply, it can be cut at birth without discomfort to either the child or woman
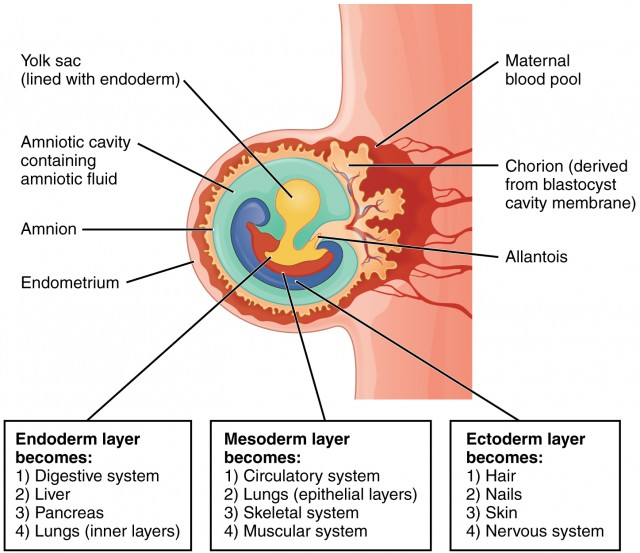
Primary Germ Layers
At the time of implantation, a blastocyst already has differentiated to a point at which two separate cavities appear in the inner structure: (1) a large one, the amniotic cavity, which is lined with a distinctive layer of cells, the ectoderm, and (2) a smaller cavity, the yolk sac, which is lined with entoderm cells. In humans, the yolk sac appears to supply nourishment only until implantation. After that, its main purpose is to provide a source of red blood cells until the embryo’s hematopoietic system is mature enough to perform this function (at about the 12th week of intrauterine life). The yolk sac then atrophies and remains only as a thin white streak discernible in the cord at birth. Between the amniotic cavity and the yolk sac, a third layer of primary cells, the mesoderm, forms. The embryo will begin to develop at the point where the three cell layers (ectoderm, entoderm, and mesoderm) meet, called the embryonic shield. Each of these germ layers of primary tissue.
Knowing the origins of body structures helps to explain why certain screening procedures are ordered for newborns with congenital malformations. A radiographic examination of the kidney, for example, may be ordered for a child born with a heart defect. A child with a malformation of the urinary tract is often investigated for reproductive abnormalities as well.
All organ systems are complete, at least in a rudimentary form, at 8 weeks gestation (the end of the embryonic period). During this early time of organogenesis (organ formation), the growing structure is most vulnerable to invasion by teratogens (any factor that adversely affects the fertilized ovum, embryo, or fetus, such as cigarette smoking).
Fetal Circulation
As early as the third week of intrauterine life, fetal blood begins to exchange nutrients with the maternal circulation across the chorionic villi. Fetal circulation differs from extrauterine circulation because the fetus derives oxygen and excretes carbon dioxide not from gas exchange in the lung but from gas exchange in the placenta.
Blood arriving at the fetus from the placenta is highly oxygenated. This blood enters the fetus through the umbilical vein (called a vein even though it carries oxygenated blood, because the direction of the blood is toward the fetal heart).Blood flows from the umbilical vein to the ductus venosus, an accessory vessel that directs oxygenated blood directly to the fetal liver.
Blood then empties into the fetal inferior vena cava so oxygenated blood is directed to the right side of the heart. Because there is no need for the bulk of blood to pass through the lungs, it is shunted, as it enters the right atrium, into the left atrium through an opening in the atrial septum, called the foramen ovale. From the left atrium, it follows the course of adult circulation into the left ventricle and into the aorta. A small amount of blood that returns to the heart via the vena cava does leave the right atrium via the adult circulatory route—that is, through the tricuspid valve into the right ventricle, and then into the pulmonary artery and lungs to service the lung tissue. However, the larger portion of even this blood is shunted away from the lungs through an additional structure, the ductus arteriosus, directly into the aorta,
MILESTONES OF FETAL GROWTH AND DEVELOPMENT

I. End of 4th Gestational Week
At the end of the fourth week of gestation, the human embryo is a group of rapidly growing cells but does not yet resemble a human being.
Length: 0.75–1 cm
Weight: 400 mg
The spinal cord is formed and fused at the midpoint.
Lateral wings that will form the body are folded forward to fuse at the midline.
The head folds forward and becomes prominent, representing about one-third of the entire structure.
The back is bent so that the head almost touches the tip of the tail.
The rudimentary heart appears as a prominent bulge on the anterior surface.
Arms and legs are budlike structures.
Rudimentary eyes, ears, and nose are discernible.
II. End of 8th Gestational Week
Length: 2.5 cm (1 in)
Weight: 20 g
Organogenesis is complete.
The heart, with a septum and valves, is beating rhythmically.
Facial features are discernible.
Arms and legs have developed.
External genitalia are forming, but sex is not yet distinguishable by simple observation.
The primitive tail is regressing.
The abdomen bulges forward because the fetal intestine is growing so rapidly.
An ultrasound shows a gestational sac, diagnostic of pregnancy (Fig. 9.8).
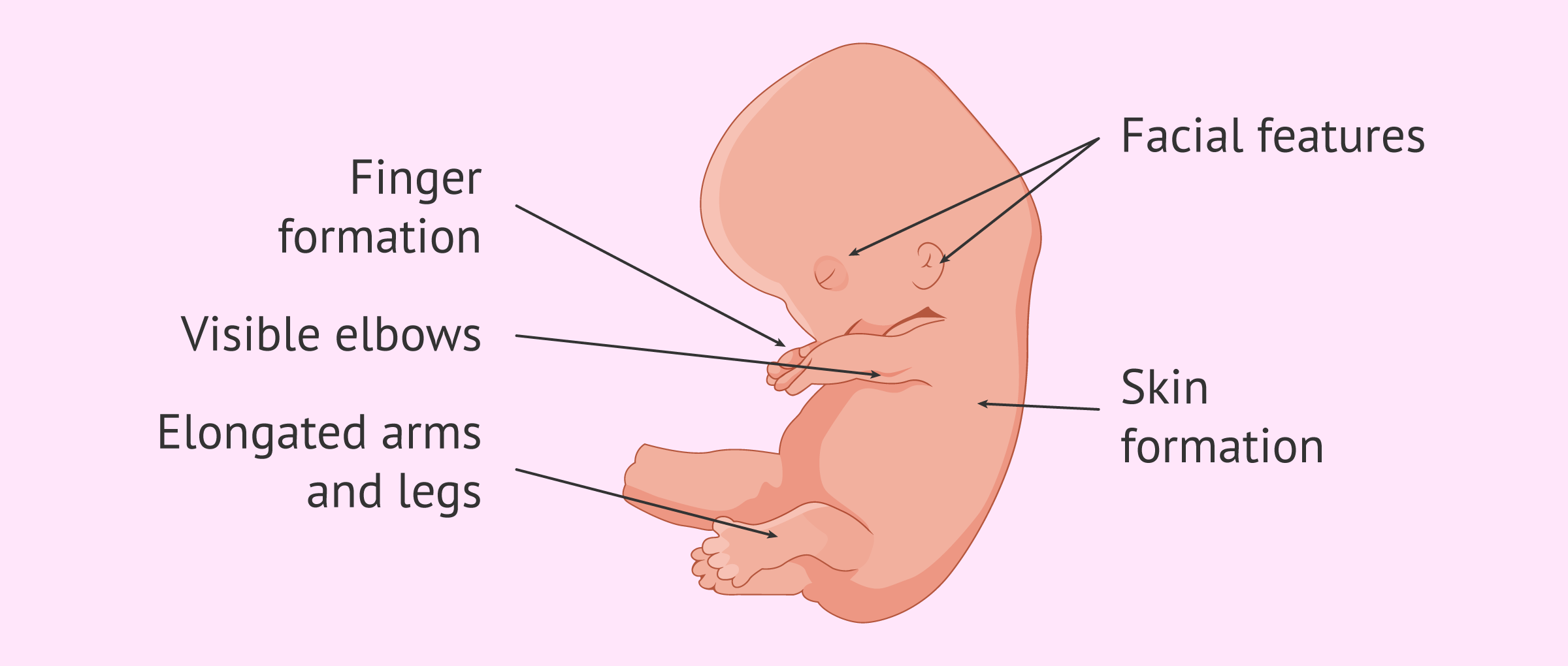
III. End of 12th Gestational Week (First Trimester)
Length: 7–8 cm
Weight: 45 g
Nail beds are forming on fingers and toes.
Spontaneous movements are possible, although they are usually too faint to be felt by the mother.
Some reflexes, such as the Babinski reflex, are present.
Bone ossification centers begin to form.
Tooth buds are present.
Sex is distinguishable by outward appearance.
Urine secretion begins but may not yet be evident in amniotic fluid.
The heartbeat is audible through Doppler technology.

IV. End of 16th Gestational Week
Length: 10–17 cm
Weight: 55–120 g
Fetal heart sounds are audible by an ordinary stethoscope.
Lanugo is well-formed
is very thin, soft, usually unpigmented hair that is sometimes found on the body of a fetus or newborn
Liver and pancreas are functioning.
The fetus actively swallows amniotic fluid, demonstrating an intact but uncoordinated swallowing reflex; urine is present in amniotic fluid.
Sex can be determined by ultrasound.
V. End of 20th Gestational Week
Length: 25 cm
Weight: 223 g
Spontaneous fetal movements can be sensed by the mother.
Antibody production is possible.
The hair forms on the head, extending to include eyebrows.
Meconium is present in the upper intestine.
a newborn's first poop, sticky, thick, dark green poop is made up of cells, protein, fats, and intestinal secretions, like bile.
Brown fat, a special fat that will aid in temperature regulation at birth, begins to form behind the kidneys, sternum, and posterior neck.
Vernix caseosa begins to form and cover the skin.
a physiological, viscous biofilm that is produced by desquamated fetal skin and sebaceous glands covering the fetus at the third trimester in-utero
Passive antibody transfer from mother to fetus begins.
Definite sleeping and activity patterns are distinguishable (the fetus has developed biorhythms that will guide sleep/wake patterns throughout life).
VI. End of 24th Gestational Week (Second Trimester)
Length: 28–36 cm
Weight: 550 g
Meconium is present as far as the rectum
Active production of lung surfactant begins.
Eyebrows and eyelashes become well-defined.
Eyelids, previously fused since the 12th week, are now open.
Pupils are capable of reacting to light.
When fetuses reach 24 weeks or 601g, they have achieved a practical low-end age of viability (the earliest age at which fetuses could survive if born at that time), if they are cared for after birth in a modern intensive care facility.
Hearing can be demonstrated by the response to sudden sounds.
VII. End of 28th Gestational Week
Length: 35–38 cm
Weight: 1200 g
Lung alveoli begin to mature, and surfactant can be demonstrated in amniotic fluid.
Testes begin to descend into the scrotal sac from the lower abdominal cavity.
The blood vessels of the retina are formed but thin and extremely susceptible to damage from high oxygen concentrations (an important consideration when caring for preterm infants who need oxygen).
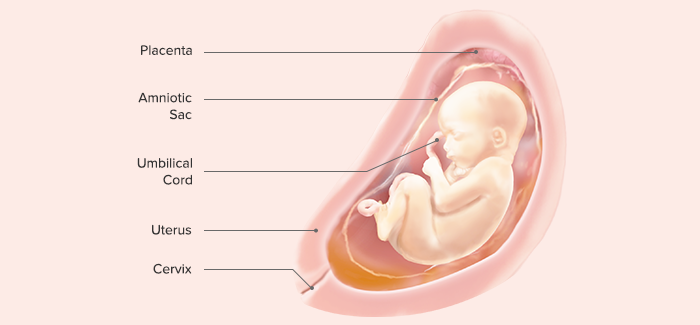
VIII. End of 32nd Gestational Week
Length: 38–43 cm
Weight: 1600 g
Subcutaneous fat begins to be deposited (the former stringy, “little old man” appearance is lost)
The fetus responds by movement to sounds outside the mother’s body.
Active Moro reflex is present.
a normal reflex for an infant when he or she is startled or feels like they are falling.
The infant will have a startled look and the arms will fling out sideways with the palms up and the thumbs flexed.
Absence of the Moro reflex in newborn infants is abnormal and may indicate an injury or disease.
Iron stores, which provide iron for the time during which the neonate will ingest only milk after birth, are beginning to be developed.
Fingernails grow to reach the end of fingertips.
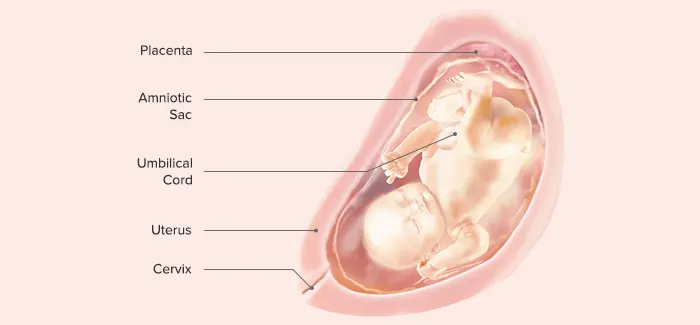
IX. End of 36th Gestational Week
Length: 42–48 cm
Weight: 1800–2700 g (5–6 lb)
Body stores of glycogen, iron, carbohydrate, and calcium are deposited.
Additional amounts of subcutaneous fat are deposited.
The sole of the foot has only one or two crisscross creases, compared with the full crisscross pattern that will be evident at term.
The amount of lanugo begins to diminish.
Most babies turn into a vertex (head down) presentation during this month.
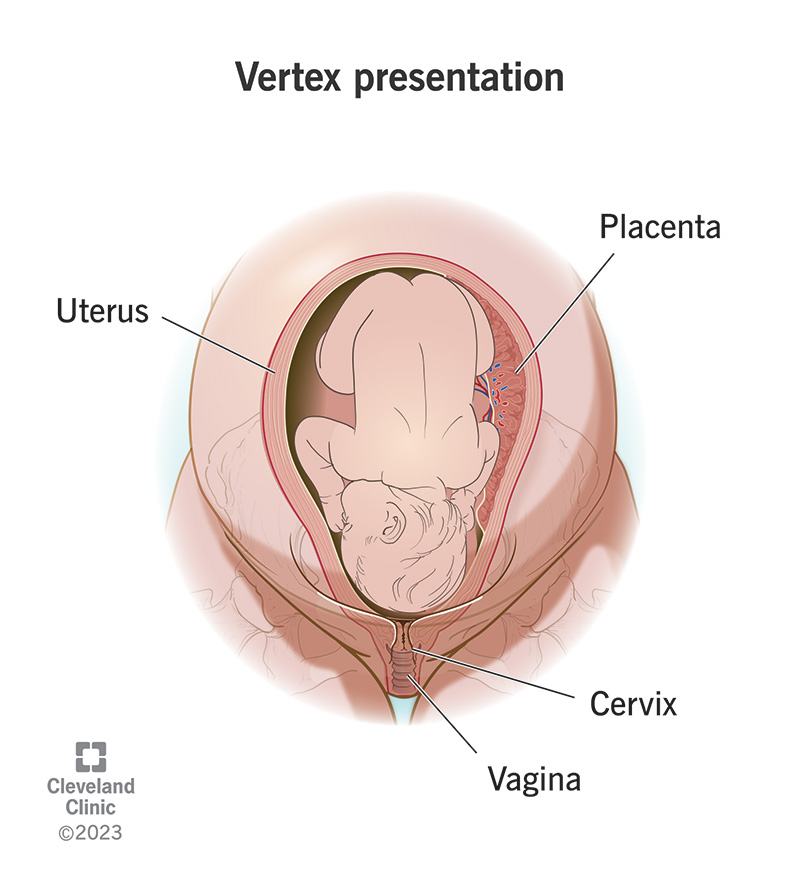
X. End of 40th Gestational Week (Third Trimester)
Length: 48–52 cm (crown to rump, 35–37 cm)
Weight: 3000 g (7–7.5 lb)
The fetus
kicks actively, hard enough to cause the mother considerable discomfort.
Fetal hemoglobin begins its conversion to adult hemoglobin. The conversion is so rapid that, at birth, about 20% of hemoglobin will be adult in character.
Vernix caseosa is fully formed.
Fingernails extend over the fingertips.
Creases on the soles of the feet cover at least two-thirds of the surface

Preventing Fetal Exposure to Teratogens
A teratogen is any factor, chemical or physical, that adversely affects the fertilized ovum, embryo, or fetus. At one time, it was assumed that a fetus in utero was protected from chemical or physical injury by the presence of the amniotic fluid and by the absence of any direct placental exchange between mother and fetus. When infants were born with disorders, it was attributed to the influence of fate, bad luck, or, in some cultures, evil spirits. Today, it is acknowledged that a fetus is extremely vulnerable to environmental injury.
Effects of Teratogens on a Fetus
Several factors influence the amount of damage a teratogen can cause. The strength of the teratogen is one of these. For example, radiation is a known teratogen. In small amounts (everyone is exposed to some radiation every day, such as from sun rays), it causes no damage. However, in large doses (e.g., the amount of radiation necessary to treat cancer of the cervix), serious fetal defects or death can occur.
The timing of the teratogenic insult makes a significant impact on the damage done to the fetus. If a teratogen is introduced before implantation, either the zygote is destroyed or it appears unaffected. If the insult occurs when the main body systems are being formed (in the second to eighth weeks of embryonic life), a fetus is very vulnerable to injury. During the last trimester, the potential for harm again decreases because all the organs of a fetus are formed and are merely maturing.
Two exceptions to the rule that deformities usually occur in early embryonic life are the effects caused by the organisms of syphilis and toxoplasmosis. These two infections can cause abnormalities in organs that were originally formed normally.
A third factor determining the effects of a teratogen is the teratogen’s affinity for specific tissue. Lead and mercury, for example, attack and disable nervous tissue. Thalidomide, a drug once used to relieve nausea in pregnancy, causes limb defects. Tetracycline, a common antibiotic, causes tooth enamel deficiencies and, possibly, long-bone deformities. The rubella virus can affect many organs: the eyes, ears, heart, and brain are the four most commonly attacked.
Teratogenic Maternal Infections
Toxoplasmosis
Toxoplasmosis, a protozoan infection, is spread most commonly through contact with uncooked meat, although it may, also be contracted through handling cat stool in soil or cat litter (Friars, 2007).
As many as 1 in 900 pregnancies may be affected by toxoplasmosis.
A woman experiences almost no symptoms of the disease except for a few days of malaise and posterior cervical lymphadenopathy. Even in light of these mild symptoms,
if the infection crosses the placenta, the infant may be born with central nervous system damage, hydrocephalus, microcephaly, intracerebral calcification, and retinal deformities.
Pre-pregnancy serum analysis can be done to identify women who have never had the disease and so are susceptible (about 50% of women).
Instruct pregnant women to avoid undercooked meat and also not to change a cat litter box or work in soil in an area where cats may defecate to avoid exposure to the disease.
If the diagnosis is established by serum analysis during pregnancy, therapy with sulfonamides may be prescribed.
Pyrimethamine, an antiprotozoal agent, may also be used. This drug is an antifolic acid drug, so it is administered with caution early in pregnancy to prevent reducing folic acid levels.
Rubella
The rubella virus usually causes only a mild rash and mild systemic illness in a woman, but the teratogenic effects on a fetus can be devastating (Johnson & Ross, 2007).
Fetal damage from maternal infection with rubella (German measles) includes hearing impairment, cognitive and motor challenges, cataracts, cardiac defects (most commonly patent ductus arteriosus and pulmonary stenosis), intrauterine growth restriction (IUGR), thrombocytopenic purpura, and dental and facial clefts, such as cleft lip and palate.
Typically, a rubella titer from a pregnant woman is obtained on the first prenatal visit. A titer greater than 1:8 suggests immunity to rubella. A titer of less than 1:8 suggests that a woman is susceptible to viral invasion. A titer that is greatly increased over a previous reading or is initially extremely high suggests that a recent infection has occurred.
A woman who is not immunized before pregnancy cannot be immunized during pregnancy because the vaccine uses a live virus that would have effects similar to those occurring with a subclinical case of rubella. After a rubella immunization, a woman is advised not to become pregnant for 3 months, until the rubella virus is no longer active.
All pregnant women should avoid contact with children with rashes. Infants who are born to mothers who had rubella during pregnancy may be capable of transmitting the disease for a time after birth. Because of this, an infant may be isolated from other newborns during the newborn period.
Cytomegalovirus
Cytomegalovirus (CMV), a member of the herpes virus family, is another teratogen that can cause extensive damage to a fetus while causing few symptoms in a woman (Lilleri et al.,2007).
It is transmitted from person to person by droplet infection such as occurs with sneezing.
If a woman acquires a primary CMV infection during pregnancy and the virus crosses the placenta, the infant may be born severely neurologically challenged (hydrocephalus, microcephaly, spasticity) or with eye damage (optic atrophy, chorioretinitis), hearing impairment, or chronic liver disease. The child’s skin may be covered with large petechiae (“blueberry-muffin” lesions).
However, diagnosis in the mother or infant can be established by the isolation of CMV antibodies in blood serum.
Unfortunately, there is no treatment for the infection even if it presents in the mother with enough symptoms to allow detection. Because there is no treatment or vaccine for the disease, routine screening for CMV during pregnancy is not recommended.
Women can help prevent exposure by thorough handwashing before eating and avoiding crowds of young children at daycare or nursery settings.
Herpes Simplex Virus (Genital Herpes Infection)
The first time a woman contracts a genital herpes infection, systemic involvement occurs. The virus spreads into the bloodstream (viremia) and crosses the placenta to a fetus posing substantial fetal risk (ACOG, 2007).
If the infection takes place in the first trimester, severe congenital anomalies or spontaneous miscarriage may occur.
If the infection occurs during the second or third trimester, there is a high incidence of premature birth, intrauterine growth restriction, and continuing infection of the newborn at birth. Unless recognized and treated, the fetal mortality and morbidity rates are as high as 80% (ACOG, 2007).
If a woman has had herpes simplex virus type 1 infections before the genital herpes invasion or if the genital herpes (type 2) infection is a recurrence, antibodies to the virus in her system prevent the spread of the virus to a fetus across the placenta.
If genital lesions are present at the time of birth, however, a fetus may contract the virus from direct exposure during birth. For women with a history of genital herpes and existing genital lesions, cesarean birth is often advised to reduce the risk of this route of infection. This awareness of the placental spread of herpes simplex virus has increased the importance of obtaining information about exposure to genital herpes or any painful perineal or vaginal lesions that might indicate this infection at prenatal visits.
Intravenous or oral acyclovir (Zovirax) can be administered to women during pregnancy (Karch, 2009). The primary mechanism for protecting a fetus, however, focuses on disease prevention. Urging women to practice safe sex is important to lessen their exposure to this and other sexually
transmitted infections. Advising adolescents to obtain a vaccine against HPV (Gardasil) should lessen the incidence of genital herpes infection in the future.
Other Viral Diseases
It is difficult to demonstrate other viral teratogens, but rubeola (measles), coxsackievirus, infectious parotitis (mumps), varicella (chickenpox), poliomyelitis, influenza, and viral hepatitis all may be teratogenic. Parvovirus B19, the causative agent of erythema infectiosum (also called fifth disease), a common viral disease in school-age children, if contracted during pregnancy, can cross the placenta and attack the red blood cells of a fetus. Infection with the virus during early pregnancy is associated with fetal death. If the infection occurs late in pregnancy, the infant may be born with severe anemia and congenital heart disease (Barankin, 2008).
A. Syphilis.
Syphilis, a sexually transmitted infection, is of great concern for the maternal-fetal population despite the availability of accurate screening tests and proven medical treatment, as it is growing in incidence and places a fetus at risk for intrauterine or congenital syphilis (Walker, 2009). Early in pregnancy, when the cytotrophoblast layer of the chorionic villi is still intact, the causative spirochete of syphilis, Treponema pallidum, cannot cross the placenta and damage
the fetus. When this layer atrophies at about the 16th to 18th week of pregnancy, however, the spirochete then can cross and cause extensive damage. If syphilis is detected and treated with an antibiotic such as benzathine penicillin in the first trimester, a fetus is rarely affected. If left untreated beyond the 18th week of gestation, hearing impairment, cognitive challenge, osteochondritis, and fetal death are possible.
For this reason, serologic screening (by either a VDRL or a rapid plasma reagin test) should be done at a first prenatal visit; the test may then be repeated close to term (the 8th month) if exposure is a concern. Even when a woman has been treated with appropriate antibiotics, the serum titer remains high for more than 200 days; an increasing titer, however, suggests that reinfection has occurred. In an infant born to a woman with syphilis, the serologic test for syphilis may remain positive for up to 3 months even though the disease was treated during pregnancy.
A newborn with congenital syphilis may have congenital anomalies, extreme rhinitis (sniffles), and a characteristic syphilitic rash, all of which identify the baby as high risk at birth (Chakraborty & Luck, 2007). When the baby’s primary teeth come in, they are oddly shaped (Hutchinson teeth).
B. Lyme Disease.
Lyme disease, a multisystem disease caused by the spirochete Borrelia burgdorferi, is spread by the bite of a deer tick. The highest incidence occurs in the summer and early fall. The largest outbreaks of the disease are found on the east coast of the United States (Mullen, 2007). After a tick bite, a typical skin rash, erythema chronicum migrans (large, macular lesions with a clear center), develops. Pain in large joints such as the knee may develop. Infection in pregnancy can result in spontaneous miscarriage or severe congenital anomalies.
To spread the spirochete, the tick must be present on the body possibly as long as 24 hours. After returning home from an outing, therefore, a woman should inspect her body carefully and immediately remove any ticks found. If she has any symptoms that suggest Lyme disease or knows she has been bitten, she should contact her primary health care provider immediately.
Treatment of Lyme disease for pregnant women differs from that for nonpregnant women. The drugs used for nonpregnant adults, tetracycline and doxycycline, cannot be used during pregnancy because they cause tooth discoloration and, possibly, long-bone malformation in a fetus. A course of penicillin will be prescribed to reduce symptoms in the pregnant woman.
Because the symptoms of Lyme disease are chronic but not dramatic (a migratory rash and joint pain), women may not report them at a prenatal visit unless they are educated about their importance and are asked at prenatal visits if such symptoms are present.
Potentially Teratogenic Vaccines
Live virus vaccines, such as measles, HPV, mumps, rubella, and poliomyelitis (Sabin type), are contraindicated during pregnancy because they may transmit the viral infection to a fetus (Rojas, Wood, & Blakemore, 2007). Care must be taken in routine immunization programs to make sure that adolescents about to be vaccinated are not pregnant. Women who work in biologic laboratories where vaccines are manufactured are well-advised not to work with live virus products during pregnancy.
Teratogenic Drugs
Many women, assuming that the rule of being cautious with drugs during pregnancy applies only to prescription drugs, take over-the-counter drugs or herbal supplements freely. Although not all drugs cross the placenta (heparin, for example, does not because of its large molecular size), most do. Also, even though most herbs are safe, ginseng, for example, used to improve general well-being or senna, used to relieve constipation, may not be safe (Der Marderosian & Beutler, 2007).
Any drug or herbal supplement, under certain circumstances, may be detrimental to fetal welfare. Therefore, during pregnancy, women should not take any drug or supplement not specifically prescribed or approved by their physician or nurse-midwife.
A woman of childbearing age and ability should not take any drug other than one prescribed
by a physician or nurse-midwife to avoid exposure to a drug should she become pregnant.
The use of recreational drugs during pregnancy puts a fetus at risk in two ways: the drug may have a direct teratogenic effect, and intravenous drug use risks exposure to diseases such as HIV and hepatitis B (Donnelly et al., 2008).
Narcotics such as meperidine (Demerol) and heroin have long been implicated as causing intrauterine growth restriction (IUGR). The use of marijuana alone apparently does not, although the long-term effects of marijuana during pregnancy are still unstudied. Cocaine, particularly its crack form, is potentially harmful to a fetus because it causes severe vasoconstriction in the mother, compromising placental blood flow and perhaps dislodging the placenta. Its use is associated with spontaneous miscarriage, preterm labor, meconium staining, and IUGR (Rojas, Wood, & Blakemore, 2007).
An area of recreational drug use that needs to be examined is that of inhalant abuse (“huffing”). Substances frequently used as inhalants include gasoline, butane lighter fluid, Freon, glue, and nitrous oxide (NIOSH, 2007).
Teratogenicity of Alcohol
Evidence over the years has shown that when women consume a large quantity of alcohol during pregnancy, their babies show a high incidence of congenital deformities and cognitive impairment. It was assumed in the past that these defects were the result of the mother’s poor nutritional status (drinking alcohol rather than eating food), not necessarily the direct result of the alcohol.
However, alcohol has now been firmly isolated as a direct teratogen. Fetuses cannot remove the breakdown products of alcohol from their bodies. The large buildup of this leads to vitamin B deficiency and accompanying neurologic damage.
Women during pregnancy should be screened for alcohol use because an infant born with fetal alcohol syndrome (FAS) not only is small for gestational age but can be cognitively challenged (Shankar, Ronis, & Badger, 2007).
Women are best advised, therefore, to abstain from alcohol completely. Be certain to ask about binge drinking (consuming more than five alcoholic drinks in an evening) as women may refer to this as only “occasional drinking.” Refer women with alcohol addiction to an alcohol treatment program as early in pregnancy as possible to help them reduce their alcohol intake.
Teratogenicity of Cigarettes
Cigarette smoking is associated with infertility in women. Cigarette smoking by a pregnant woman has been shown to cause fetal growth restriction (Lawrence & Haslam, 2007). In addition, a fetus may be at greater risk for being stillborn (Hogberg & Cnattingius, 2007) and, after birth, may be at greater risk than others for sudden infant death syndrome. Low birth weight in infants of smoking mothers results from vasoconstriction of the uterine vessels, an effect of nicotine. This limits the blood supply to a fetus.
Another contributory effect may be related to inhaled carbon monoxide. Secondary smoke, or inhaling the smoke of another person’s cigarettes, may be as harmful as actually smoking the cigarettes. All prenatal healthcare settings should be smoke-free environments for this reason.
The best way to urge women to discontinue smoking is to educate them about the risks to themselves and their fetus at a first prenatal visit. It may be effective to encourage women to sign a contract with a health care provider to try to stop or to join a smoking-cessation program.
Radiation
Rapidly growing cells are extremely vulnerable to destruction by radiation. That makes radiation a potent teratogen to unborn children because of their high proportion of rapidly growing cells. Radiation produces a range of malformations depending on the stage of development of the embryo or fetus and the strength and length of exposure. If the exposure occurs before implantation, the growing zygote is killed. If the zygote is not killed, it survives unharmed. The most damaging time for exposure and subsequent damage is from implantation to 6 weeks after conception (when many women are not yet aware that they are pregnant). The nervous system, brain, and retinal innervation are most affected.
As a rule, therefore, all women of childbearing age should be scheduled for pelvic x-ray examinations only in the first 10 days of a menstrual cycle (when pregnancy is unlikely because ovulation has not yet occurred), except in emergencies. A serum pregnancy test can be done on all women who have reason to believe they might be pregnant before diagnostic tests involving X-rays are scheduled.