3.13: Bond Enthalpies
Enthalpy of Reaction
The enthalpy change of a reaction gives the amount of heat energy released (for negative values) or absorbed (for positive values) by a chemical reaction at constant pressure. Is represented by (delta) H (subscript “rxn“); and represents the difference between reactants and products.
The (delta) H value always corresponds to the molar coefficients for the reaction, meaning:
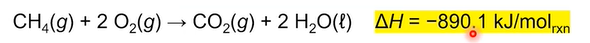
Enthalpy reactions can also be written with heat within the equation. If the equation is exothermic, the heat would be on the products side, if endothermic, then on the reactants side.
Bond energy is the energy stored in a bond; if negative, it represents the energy released when a bond forms; if positive, it represents the energy required to break a bond.
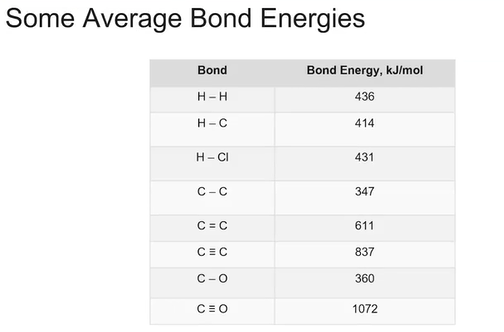
using the average bond energies, we can estimate the overall enthalpy change in a reaction.
Formula for estimating average bond energies: (delta) H (subscript “rxn“) = E(BE of bonds broken) [value will be positive] + E(BE of bonds formed) [value will be negative].