3.11 Spectroscopy and the Electromagnetic Spectrum & 3.13 Beer-Lambert Law
Chapter Summary & Important Vocab
Molecular Orbital Theory
Electromagnetic Spectrum
UV/Vis Spectroscopy
IR Spectroscopy
Microwave Spectroscopy
Hybrid Orbital Theory
Atomic orbitals on the same atom combine in order to form hybrids.
Atomic orbitals on different atoms overlap in order to form covalent bonds.
Each atom in the compound retains its associated orbitals and electrons.
This theory correlates with observed bond angles in molecules.
Molecular Orbital (MO) Theory
Views a molecule as a whole instead of a collection of individual atoms.
MO’s are similar to atomic orbitals.
They both have specific energy levels.
They both have specific sizes and shapes.
They can both hold a maximum of two electrons that spin in opposite directions.
Atomic orbitals combine to for MO’s.
When two atomic orbitals combine, two MO’s are formed.
Orbitals are always ‘conserved’.
Molecular Orbitals
Bonding Orbital
A MO that is lower in energy than any atomic orbital from which it was derived.
Electrons that occupy these orbitals cause stability.
Anti-Bonding Orbital
A MO that is higher in energy than any atomic orbitals from which it was derived.
Electrons that occupy these orbitals cause instability.
Non-Bonding Orbital:
A MO that is the same energy level as the one atomic orbital that it was derived from.
Electrons that occupy these orbitals do not cause stability or instability.
Orbitals that contain lone pairs.
The Electromagnetic Spectrum
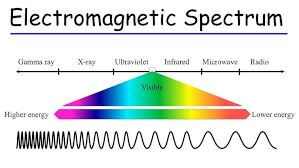
Spectroscopy
Spectroscopy:
A Method of analysis which is based upon the absorbance of electromagnetic radiation by matter.
Used to acquire data pertaining to the structure of a molecule or the concentration of a species.
Io = It + Ia
Io - Intensity of electromagnetic radiation striking sample
It - Intensity of electromagnetic radiation exiting sample
Ia - Intensity of electromagnetic radiation absorbed by the sample.
Two methods used for Spectroscopic analysis:
1. Ultraviolet/Visual (UV/Vis) Spectroscopy
Examines transitions in the electronic energy levels
Is used to probe the electronic structure of certain compounds.
Is used to determine concentrations of solutions that contain certain compounds.
2. Infrared (IR) Spectroscopy
Examines transitions in molecular vibrations
Is used to detect the presence of different types of bonds and to identify molecules.
Ultraviolet/Visual (UV/Vis) Spectroscopy
An absorption spectrometer is used to measure the absorbance of a sample at wavelengths between about 200 nm and 800 nm.
The peaks represent wavelengths that correspond to the energy associated with possible electronic transitions within the molecule.
Colorless species can only absorb UV light between about 200 nm and 400 nm in these experiments.
Colored species will always absorb light from the visual spectrum, but could also absorb UV light in these experiments.
Beer-Lambert Law
A = Ebc
A = Absorbance
E = Molar Absorptivity
b = Path Length
C = Concentration (M)
E describes how intensely a sample of ions or molecules absorbs light at a specific wavelength.
In most experiments, the path length and wavelength remain constant, so E also remains constant and absorbance A is only proportional to concentration, c.
Infrared (IR) Spectroscopy
All covalent bonds in molecules are vibrating.
Bond length is the average distance between nuclei.
Covalent bonds have a vibrational frequency that is the IR region of the electromagnetic spectrum.
IR radiation of exactly the same frequency will be absorbed by the molecule.
Vibrational frequencies depend on the mass of the atoms and the strength of the bonds.
Frequency is related to wavelength -c = h x v
IR Spectra can be used to identify bond types, functional groups, and compounds.
Molecules that share the same functional group, such as alcohols (-OH) or carboxylic acids (-COOH) can be identified through IR spectroscopy, as they will have absorption peaks within the same range.
Every compound has a characteristic IR spectrum that it can be identified through.
Microwave Spectroscopy
Microwaves cause polar molecules to rotate.
Each type of polar molecules has specific rotational frequencies that it can exhibit.
The peaks in the microwave spectra blow correlate with the different rotational frequencies for a specific polar molecule.
Data from microwave spectra can be used to calculate the bond length or diatomic polar molecules and to determine the shapes of polar molecules.