
Cytotoxic T cells
3: T cells develop in the thymus. They’re CD3+ and leave the thymus fully mature. CD3 is necessary for signal transduction once the presented antigen is encountered. T cells are long-lived and slowly self renew in peripheral lymphoid tissues. Maintained by self peptide: self MHC complex.
4: CD4 (Th cells) + MHC II, CD8 (Tc cells) + MHC I
5: Double positive (DP) thymocyte may express a TCR which interacts with MHC II on cortical thymic epithelial cell (cTEC) (??). CD4 co-receptor assists interaction → loss of uninvolved coreceptor CD8 expression, or other way round.
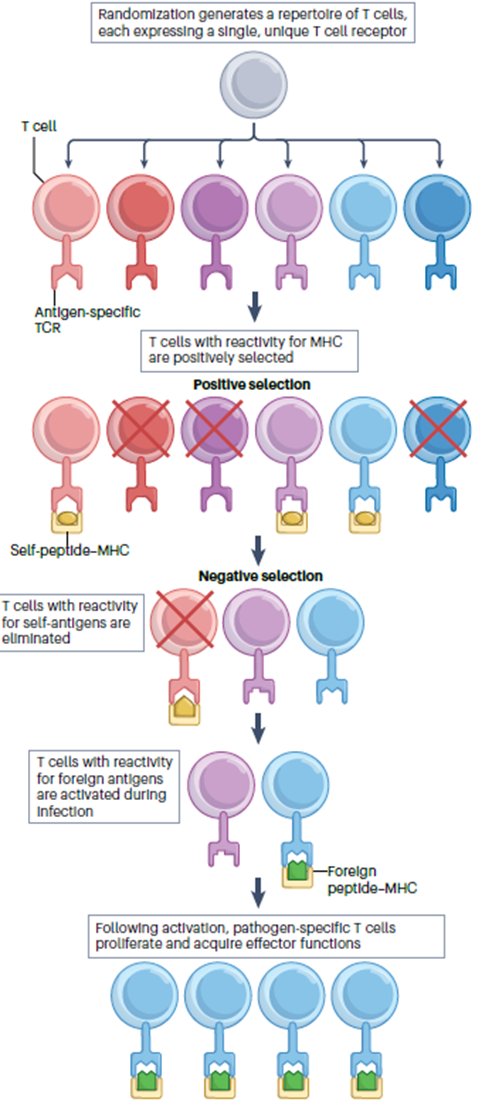
6: Elimination of infected cells without destroying healthy tissue requires CD8 T cells to be both powerful and accurate. T cells must be activated to proliferate and differentiate into effector cells before they can function. This occurs in peripheral lymphoid organs on surface of antigen presenting cells which present MHC complexed antigen peptides.
7: Cytotoxic T cells target infected cells. Intracellular pathogens aren’t accessible to antibodies so can only be eliminated by destruction of the infected cells they reside on
cytosolic pathogens | intravesicular cells | extracellular pathogens and toxins | |
degraded in | cytosol | endocytic vesicles (low pH) | endocytic vesicles (low pH) |
peptides bind | MHC class I | MHC class II | MHC class II |
presented to | effector CD8 | effector CD4 | effector CD4 |
effect on presenting cell | cell death | activation to kill intravesicular bacteria and parasites | B cell activation to secrete antibodies |
8:
MHC I:
on all nucleated cells
intracellular peptides for presentation made by cytosolic proteases and proteasome
transported → ER
peptide assembled with ER and transported to cell surface
MHC II:
only on APCs
present extracellular antigens
exocytosis of exogenous antigen
9: MHC I consists of 2 polypeptide chains: alpha and beta2-microglobulin. MHC-I alpha chains consist of a single polypeptide made up of 3 extracellular domains and a transmembrane region which anchors it. Beta2-microglobulin non-covalently bound to alpha chains. Alpha anchors it to membrane
The alpha 1 and 2 domains fold together into a structure of two segmented alpha helices lying on a sheet of 8 antiparallel bets strands which create a long cleft/groove where peptide antigens bind. The cleft contains a series of pockets, A to F which have different chemical properties between different allomorphs (different T cells?)
10: Antigen presented on MHCs recognised by TCRs which are antigen specific and their diversity is achieved by V(D)J recombination.
11: Cytotoxic T cells carry out targeted cell killing where cells presenting antigen-MHC-I complexes are instructed to die. Mediated by soluble factors. T cells operate in a dense system packed with bystander cells.
Tumour cells arise due to genetic mutation and transformation of normal cells. Adjacent healthy cells need to be protected to limit wide destruction. This is crucial to maintaining tissue integrity
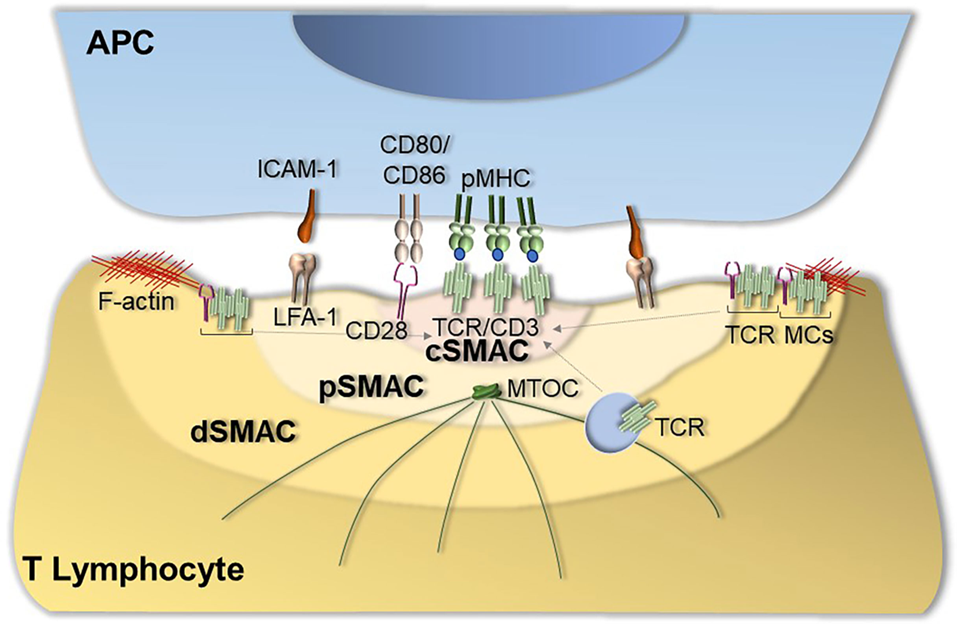
12: Immunological synapses form between the T cell and the APC. They consist of a central cluster containing the TCR surrounded by a peripheral ring enriched in LFA-1/CD11b. The synapse allows T cells to be highly selective of which surrounding cells receive signals.
14:
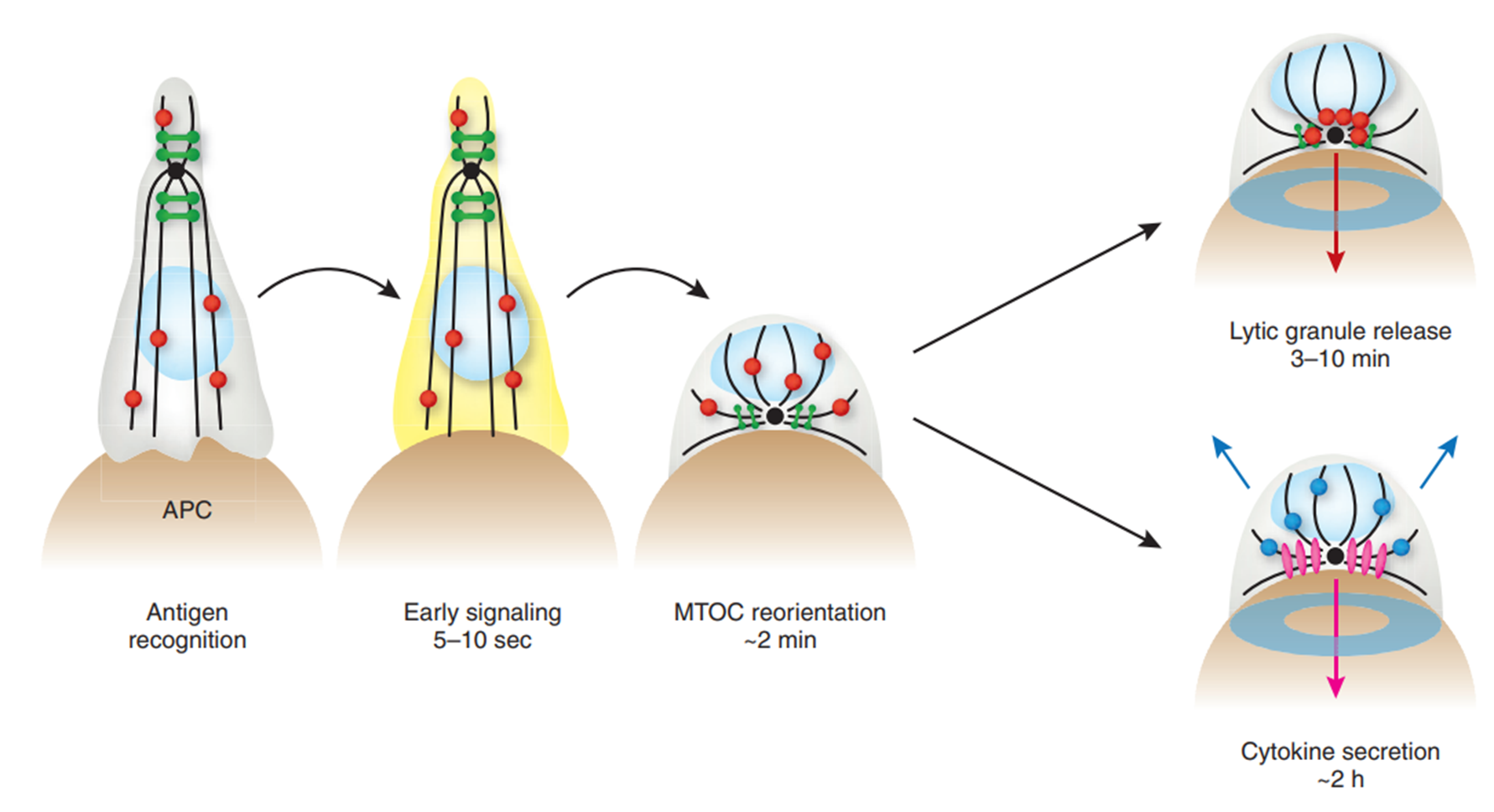
Antigen recognition triggers early signalling responses within 10 seconds, followed by reorientation of microtubule-organising centre (MTOC). This enables synapse formation and lytic granule release.
Microtubules radiate with defined polarity from the MTOC - minus ends directed in and plus ends directed out. Allows central binding zone to be recognised. Within 10 minutes, MTOC is polarised to just below IS.
15: As CD8+ T cells mature, cytolytic factors eg perforin and granzyme (main mediators of cytotoxic mechanisms) are compartmentalised into lytic granules - acidified secretory lysozymes. Granules attached to microtubule cytoskeleton. After MToc polarisation, lytic granules traffic towards the MTOC. Individual lytic granules are extruded in a specific domain at IS centre. The secretory domain is found at the peripheral integrin ring and is adjacent to the central cluster of TCRs.
Release of lytic granules into the cleft allows specific killing of the target cell by limiting the exposure of surrounding cells to granule contents.
16: Apoptosis is a form of programmed cell death where the cell activate an internal death program.
Characterised by nuclear DNA degradation, nuclear degradation and condensation, shedding of cell contents and membrane blebbing. Occurs naturally in development. Proliferating lymphocytes undergo high apoptosis rates in development.
17: Triggering cytotoxicity causes perforin release into the synapse. Perforin is a calcium dependent, pore-forming protein which multimerises in membranes. It delivers granzymes into target cytosol. It was originally hypothesised to form pores in the membrane which granzymes pass through however the pores are too small.
18: An alternative hypothesis for triggering apoptosis is that perforin forms small pores in the membrane which trigger calcium influx.
Influx activates membrane repair response where internal vesicles donate their membranes to patch holes. rapid co-endocytosis of granzymes and perforin into giant endosomes occurs and there’s perforin-mediated granzyme release into the cytosol.
Familial hemophagocytic lymphohistocytosis (FHL) is caused by biallelic perforin mutation (can’t generate perforin) → severely immunocompromised.
19: Granzymes are a family of highly homologous serine proteases found in cytotoxic granules of both innate and adaptive immune killer cells. Five human granzymes, encoded from 3 gene clusters. Granzymes are homologous to trypsin and their overall structure is similar to other related serine proteases. GzmA and B are most abundant and B is the most well studied.
20: Granzyme B activates caspase pathway by cleaving caspase 3, and it proteolyses many of the important caspase substrates directly. The pro-apoptotic cascade activates BID which oligomerises BAK or BAX into pores which result in cytochrome C release.
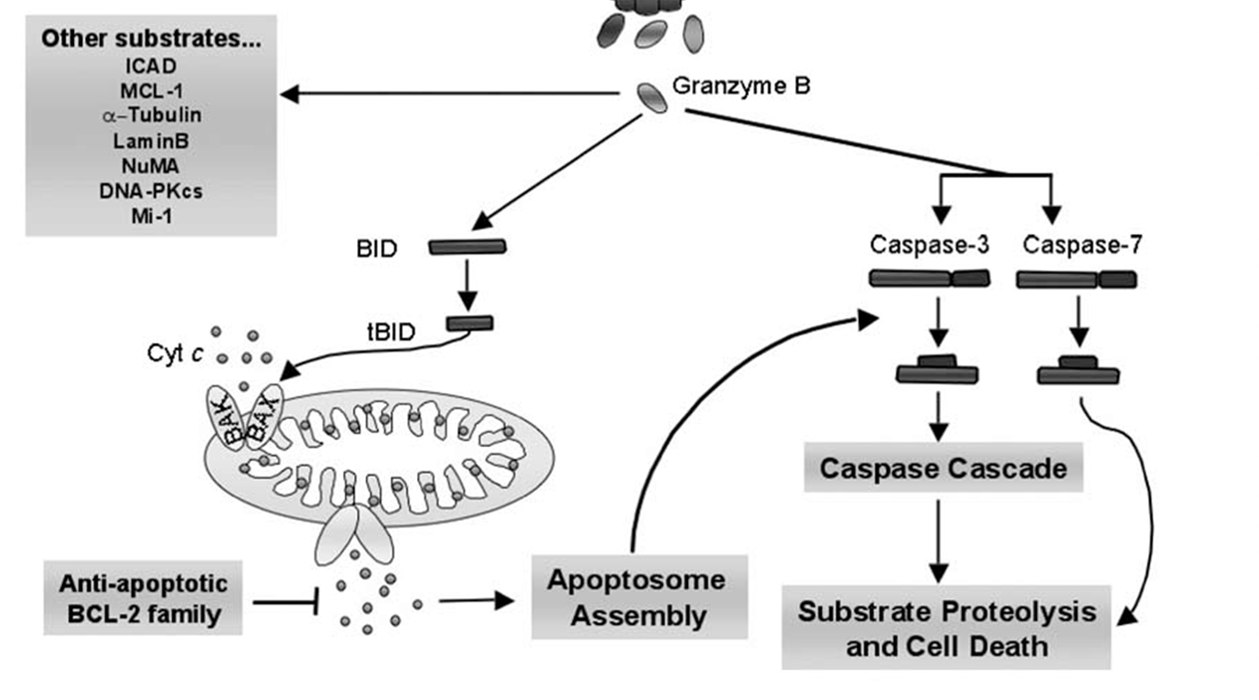
21: Granzyme A is caspase independent and damages DNA and mitochondria directly.
DNA:
rapidly translocates to nucleus, mediated by importin alpha
damages DNA by causing ss nicks via DNAse → apoptosis
activates TREX1 to prevent DNA annealing and repairing
mitochondria:
traffics into mitochondrial matrix
initiates mitochondrial damage
Cell death initiated by GzmA have all morphological features of apoptosis but caspase independent and involves novel pathways
22: Granzyme H = induces caspase and BID independent cell death via chromosomal condensation and nuclear fragmentation
Granzyme C = mitochondrial depolarisation and cytochrome C release
K = induces ROS, may kill like A
23: Entire process completed in minutes and doesn’t exhaust complement of lytic granules. Cytotoxic T cells can undergo repeated cycles of antigen recognition, polarisation and cytolysis.
TCR ligation induces de novo perforin and granzyme synthesis in armed effector CD8 T cells replenishing lytic granule supply.
IL2 regulates perforin and granzyme expression directly and independently of its effect on CD8+ T cell survival and proliferation. Individual CD8 T cells can kill many targets in succession.
24: perforin binds inhibitor calreticulin upon ER synthesis. Perforin inactive at acidic pH of cytolytic granules and is complexed with proteoglycan inhibitor serglycin for stability. Perforin is only activated in neutral pH therefore doesn’t damage cytotoxic T cells. Membrane associated cathepsin B can cleave perforin to protect activated cytotoxic T cell and precents retrograde destructive action on T cells.
3: T cells develop in the thymus. They’re CD3+ and leave the thymus fully mature. CD3 is necessary for signal transduction once the presented antigen is encountered. T cells are long-lived and slowly self renew in peripheral lymphoid tissues. Maintained by self peptide: self MHC complex.
4: CD4 (Th cells) + MHC II, CD8 (Tc cells) + MHC I
5: Double positive (DP) thymocyte may express a TCR which interacts with MHC II on cortical thymic epithelial cell (cTEC) (??). CD4 co-receptor assists interaction → loss of uninvolved coreceptor CD8 expression, or other way round.

6: Elimination of infected cells without destroying healthy tissue requires CD8 T cells to be both powerful and accurate. T cells must be activated to proliferate and differentiate into effector cells before they can function. This occurs in peripheral lymphoid organs on surface of antigen presenting cells which present MHC complexed antigen peptides.
7: Cytotoxic T cells target infected cells. Intracellular pathogens aren’t accessible to antibodies so can only be eliminated by destruction of the infected cells they reside on
cytosolic pathogens | intravesicular cells | extracellular pathogens and toxins | |
degraded in | cytosol | endocytic vesicles (low pH) | endocytic vesicles (low pH) |
peptides bind | MHC class I | MHC class II | MHC class II |
presented to | effector CD8 | effector CD4 | effector CD4 |
effect on presenting cell | cell death | activation to kill intravesicular bacteria and parasites | B cell activation to secrete antibodies |
8:
MHC I:
on all nucleated cells
intracellular peptides for presentation made by cytosolic proteases and proteasome
transported → ER
peptide assembled with ER and transported to cell surface
MHC II:
only on APCs
present extracellular antigens
exocytosis of exogenous antigen
9: MHC I consists of 2 polypeptide chains: alpha and beta2-microglobulin. MHC-I alpha chains consist of a single polypeptide made up of 3 extracellular domains and a transmembrane region which anchors it. Beta2-microglobulin non-covalently bound to alpha chains. Alpha anchors it to membrane
The alpha 1 and 2 domains fold together into a structure of two segmented alpha helices lying on a sheet of 8 antiparallel bets strands which create a long cleft/groove where peptide antigens bind. The cleft contains a series of pockets, A to F which have different chemical properties between different allomorphs (different T cells?)
10: Antigen presented on MHCs recognised by TCRs which are antigen specific and their diversity is achieved by V(D)J recombination.
11: Cytotoxic T cells carry out targeted cell killing where cells presenting antigen-MHC-I complexes are instructed to die. Mediated by soluble factors. T cells operate in a dense system packed with bystander cells.
Tumour cells arise due to genetic mutation and transformation of normal cells. Adjacent healthy cells need to be protected to limit wide destruction. This is crucial to maintaining tissue integrity
12: Immunological synapses form between the T cell and the APC. They consist of a central cluster containing the TCR surrounded by a peripheral ring enriched in LFA-1/CD11b. The synapse allows T cells to be highly selective of which surrounding cells receive signals.

14:

Antigen recognition triggers early signalling responses within 10 seconds, followed by reorientation of microtubule-organising centre (MTOC). This enables synapse formation and lytic granule release.
Microtubules radiate with defined polarity from the MTOC - minus ends directed in and plus ends directed out. Allows central binding zone to be recognised. Within 10 minutes, MTOC is polarised to just below IS.
15: As CD8+ T cells mature, cytolytic factors eg perforin and granzyme (main mediators of cytotoxic mechanisms) are compartmentalised into lytic granules - acidified secretory lysozymes. Granules attached to microtubule cytoskeleton. After MToc polarisation, lytic granules traffic towards the MTOC. Individual lytic granules are extruded in a specific domain at IS centre. The secretory domain is found at the peripheral integrin ring and is adjacent to the central cluster of TCRs.
Release of lytic granules into the cleft allows specific killing of the target cell by limiting the exposure of surrounding cells to granule contents.
16: Apoptosis is a form of programmed cell death where the cell activate an internal death program.
Characterised by nuclear DNA degradation, nuclear degradation and condensation, shedding of cell contents and membrane blebbing. Occurs naturally in development. Proliferating lymphocytes undergo high apoptosis rates in development.
17: Triggering cytotoxicity causes perforin release into the synapse. Perforin is a calcium dependent, pore-forming protein which multimerises in membranes. It delivers granzymes into target cytosol. It was originally hypothesised to form pores in the membrane which granzymes pass through however the pores are too small.
18: An alternative hypothesis for triggering apoptosis is that perforin forms small pores in the membrane which trigger calcium influx.
Influx activates membrane repair response where internal vesicles donate their membranes to patch holes. rapid co-endocytosis of granzymes and perforin into giant endosomes occurs and there’s perforin-mediated granzyme release into the cytosol.
Familial hemophagocytic lymphohistocytosis (FHL) is caused by biallelic perforin mutation (can’t generate perforin) → severely immunocompromised.
19: Granzymes are a family of highly homologous serine proteases found in cytotoxic granules of both innate and adaptive immune killer cells. Five human granzymes, encoded from 3 gene clusters. Granzymes are homologous to trypsin and their overall structure is similar to other related serine proteases. GzmA and B are most abundant and B is the most well studied.
20: Granzyme B activates caspase pathway by cleaving caspase 3, and it proteolyses many of the important caspase substrates directly. The pro-apoptotic cascade activates BID which oligomerises BAK or BAX into pores which result in cytochrome C release.

21: Granzyme A is caspase independent and damages DNA and mitochondria directly.
DNA:
rapidly translocates to nucleus, mediated by importin alpha
damages DNA by causing ss nicks via DNAse → apoptosis
activates TREX1 to prevent DNA annealing and repairing
mitochondria:
traffics into mitochondrial matrix
initiates mitochondrial damage
Cell death initiated by GzmA have all morphological features of apoptosis but caspase independent and involves novel pathways
22: Granzyme H = induces caspase and BID independent cell death via chromosomal condensation and nuclear fragmentation
Granzyme C = mitochondrial depolarisation and cytochrome C release
K = induces ROS, may kill like A
23: Entire process completed in minutes and doesn’t exhaust complement of lytic granules. Cytotoxic T cells can undergo repeated cycles of antigen recognition, polarisation and cytolysis.
TCR ligation induces de novo perforin and granzyme synthesis in armed effector CD8 T cells replenishing lytic granule supply.
IL2 regulates perforin and granzyme expression directly and independently of its effect on CD8+ T cell survival and proliferation. Individual CD8 T cells can kill many targets in succession.
24: perforin binds inhibitor calreticulin upon ER synthesis. Perforin inactive at acidic pH of cytolytic granules and is complexed with proteoglycan inhibitor serglycin for stability. Perforin is only activated in neutral pH therefore doesn’t damage cytotoxic T cells. Membrane associated cathepsin B can cleave perforin to protect activated cytotoxic T cell and precents retrograde destructive action on T cells.