Antihistamines
Antihistamines
What are Allergies?
An allergy is a response by your body to something that is ordinarily harmless. The purpose of the immune system is to recognize harmful infection-causing invaders and to get rid of them. However, for some people, the immune system overreacts to ordinarily harmless substances like indoor allergens such as pet dander, pollen, dust, mold and outdoor allergens such as pollen from grass, trees and weeds. These people have “allergies.” Their bodies attempt to expel these ordinarily harmless substances, causing sneezing; a runny nose; itchy, watery eyes and sometimes hives.
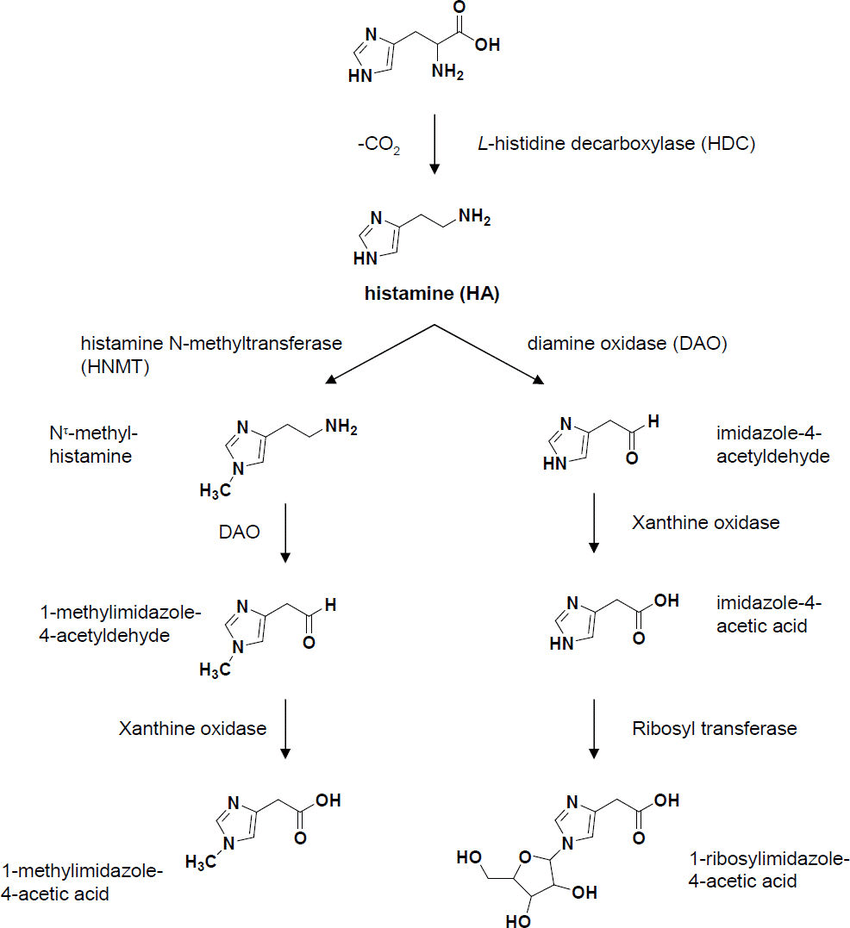
Histamine
4-(2-aminoethyl) imidazole
2-(4-imidazoyl) ethylamine
Present in the mast cells of many body organs, in blood basophils, the mucosal cells of the gastrointestinal tract especially the acid-secreting parietal cells, in the hypothalamus and area postrema in the central nervous system.
Biosynthesis
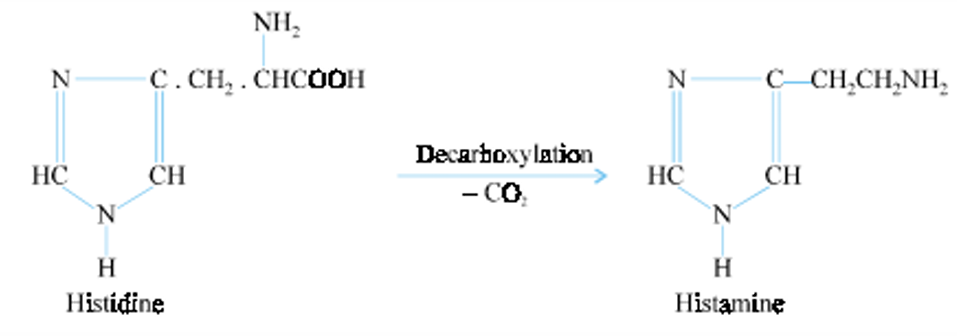
Histamine Receptors
Agonist | Receptor | Location | Effect |
|---|---|---|---|
2-Methylhistamine | H1 | CNS Blood vessels, endothelial cells Bronchi Ileum Heart Adrenals Nose, bronchi | cGMP increases, Headache; wakefulness, Arterial vasodilation Postcapillary venules constriction Smooth muscle contraction Smooth muscle contraction Increased coronary blood flow Release of catecholamines Increased exocrine secretion |
4-Methylhistamine | H2 | Heart Mast cells Stomach Blood vessels, smooth muscles | Chronotropy, inotropy Negative feedback Acid production Slow drop in pressure |
a-Methylhistamine | H3 | Brain Airways, neurons GI tract | Negative feedback on histamine release (sedative action) Inhibitory action on some forms of bronchoconstriction Acetylcholine release inhibited |
Antihistamines
Antihistamines are drugs that block the action of histamine at the H1 receptor sites, responsible for immediate hypersensitivity reactions such as sneezing and itching. Members of this class of drugs may also be used for their side effects, including sedation and antiemesis (n of nausea and vomiting. H1 antihistamines antagonize all actions of histamine except for those preventiomediated by H2 receptors. The H2 receptor antagonists are the drugs that inhibit competitively the interaction of histamine with H2 receptors. H2 receptor antagonists inhibit gastric acid secretion induced by histamine thus they are used to treat gastric and duodenal ulcers.
Antihistamines do not cure allergies or prevent histamine from being released. They also have no effect on other chemicals that the body releases when exposed to allergens. For these reasons, antihistamines can be expected to reduce allergy symptoms by only about 50%. In some people antihistamines become less effective when used over a long time. Sometimes switching to another type of antihistamine may help to cure reactions.
Antihistaminics are widely used in the palliative treatment in allergic conditions like hay fever, urticaria, some forms of pruritus, rhinitis, conjunctivitis, nasal discharge, mild asthma etc. A few antihistaminics possess potent antiemetic action and hence are frequently employed in the prevention and treatment of irradiation sickness, motion sickness (air, sea, road), nausea in pregnancy and postoperative vomiting.
How do Antihistamines Work?
An antihistamine is not actually the opposite of a histamine. An antihistamine is a drug that binds to certain sites (receptors on cells) in the body, in order to prevent histamine from binding.
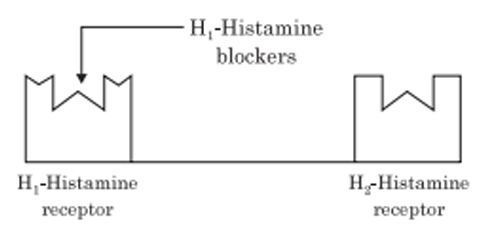
Antihistamines act as competitive inhibitors of histamine (agonist) binding. Antihistamines compete for the H1 receptors on blood vessel endothelial cells and smooth muscle cells. As competitive inhibitors, the antihistamines bind to the receptors because there is more of the antihistamine than the natural histamine. Antihistamines have no effect on the rate of histamine release, nor do they inactivate histamine.
Classification
First Generation/Classical Antihistamines
Aminoalkylethers/Ethanolamines: Diphenhydramine, Bromodiphenhydramine, Dimenhydrinate, Doxylamine, Diphenylpyraline, Carbinoxamine
Ethylenediamines: Tripelennamine, Clestamine, Pyrilamine, Mepyramine, Thonzylamine, Zolamine
Thiophene Derivatives: Methapyrilene, Methaphenilene, Thenyldiamine, Chlorothen
Dibenzocycloheptenes: Cyproheptadine, Azatidine
Alkylamines/Propylamine Derivatives: Chlorpheniramine, Pheniramine, Dexchlorpheniramine, Brompheniramine, Dexbrompheniramine, Tripolidine, Phenylpropanolamine
Piperazine Derivatives: Cyclizine, Chlorcyclizine, Meclizine, Buclizine
Phenothiazine Derivatives: Promethazine, Trimeprazine, Methdilazine
Second Generation Antihistamines
Piperazine Derivatives: Cetirizine, Levocetirizine
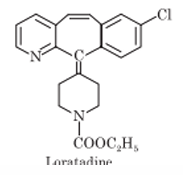
Piperidine Derivatives: Loratadine, Desloratadine, Fexofenadine
Miscellaneous/Newer: Bilastine, Ebastine, Phenidamine, Dimenthindene, Antazoline
Structure Activity Relationships
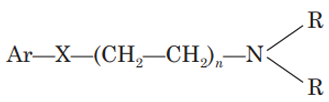
The nitrogen should be 3° in nature for maximum antihistaminic activity. The ‘N’ may also form a part of heterocyclic moieties like piperidine, or piperazine or diazocine. The nitrogen amine should be separated by 5-6 A° from the aromatic or heteroaromatic group. (N in the structure)
The group present between nitrogen atom and group X may be saturated or unsaturated or substituted. ((CH2—CH2)n in the structure)
The Ar group may be aryl or heteroaryl, which may be substituted. (Ar in the structure)
The group (X) can be carbon, oxygen or nitrogen. (X in the structure)
Aminoalkyl Ether Derivatives
The basic structure of aminoalkyl ethers for H1 antihistaminic activity:
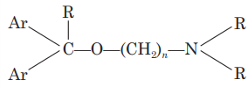
The aromatic groups may be phenyl or substituted phenyl or heterocyclic for good antihistaminic activity. The R group may be methyl or hydrogen.
If double bond is introduced between α, β-carbon atoms of propyl chain, drowsiness will be developed Ex: acrilestine.
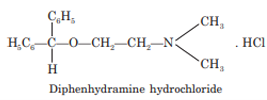
Diphenhydramine
Diphenhydramine is chemically an ethanolamine, and in addition to its role in reducing allergic reactions, may be used as a night time sedative, for control of drug-induced parkinsonism, and, in liquid form, for control of coughs. Diphenhydramine occurs as hydrochloride salt which is 2-benzhydryloxyethyldimethyl hydrochloride.
 pppp
pppp
Carbinoxamine
Chemistry: Carbinoxamine is available as carbinoxamine maleate. Chemically it is 2-[-p-chloro-(-(2-(dimethylamino) ethoxy] benzyl] pyridine maleate.
Properties: It occurs as white, odorless, crystalline powder. It is freely soluble in water, alcohol, and chloroform.
Synthesis: Piconaldehyde by Grignard reaction with p
chlorophenylmagensium bromide gives an intermediate which is converted to carbinoxamine by adding N-dimethylaminoethyl chloride in presence of sodamide.
Uses: Carbinoxamine is an antihistamine with anticholinergic and antiemetic activities. It is effective in allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis.

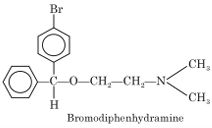
Bromodiphenhydramine
Chemistry: Bromodiphenhydramine is 2[(4-bromophenyl) phenylmethoxy)]-N, N-dimethylethanamine.
Properties: It occurs as hydrochloride salt. Bromodiphenhydramine HCl is a white crystalline, water-soluble powder.
Dimenhydrinate
Dimenhydrinate is aminoalkyl ether. It is 8-chlorotheophyllinate salt of diphenhydramine. Dimenhydrinate is a white crystalline, colorless, slightly water-soluble powder. It is used to treat motion-sickness, nausea and vertigo.
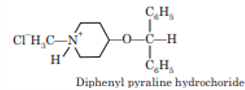
Diphenylpyraline
Chemistry: Diphenylpyraline is a piperidine derivative, which occurs as hydrochloride salt. Chemically it is 4-(diphenylmethoxy)-1-methylpiperidine.
Properties: Diphenylpyraline hydrochloride is water-soluble white crystalline powder. It is soluble in alcohol but insoluble in ether and benzene
Synthesis: Diphenylpyraline is synthesized by refluxing the mixture of 1-methyl-4- piperidinol and benzhydryl bromide.
Uses: It is used as an antihistaminic agent. It is effective for perennial and seasonal allergic rhinitis, vasomotor rhinitis, allergic conjunctivitis. It also possesses anticholinergic and sedative effects.
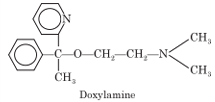
Doxylamine
Chemistry: Doxylamine occurs as doxylamine succinate. Chemically it is 2-[(-[2- (dimethylamino) ethoxy]-(-methylbenzyl] pyridine succinate. Doxylamine succinate is white, characteristic odor, water or alcohol soluble powder.
Uses: Doxylamine is an antihistaminic agent but it has sedative effect. It is also used with antitussives and decongestants for the relief of cough and cold.
Clemastine
Chemistry: Clemastine is an alkyl ether derivative possessing of pyrrolidine nucleus. It occurs as clemastine fumarate. Chemically, clemastine is 2-[2-[1-(p-chlorophenyl)-1- phenylethoxy]ethyl]-1-methylpyrrolidine.
Properties: Clemastine fumarate is white or slightly yellow colored, crystalline powder. It is slightly soluble in water and alcohol.
Uses: Clemastine has antihistaminic activity with anticholinergic and sedative effects.
Ethylenediamines
The basic structure of ethylene diamine derivatives of H1 antagonists is:
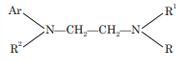
R and R1 are methyl groups, R2 may be phenyl, benzyl or heterocyclic and Ar may be phenyl or heterocyclic for antihistaminic activity.
All compounds of this series have two 3° nitrogens which are separated by a two carbon atom chain. Substitution of heterocyclic groups for Ar or introduction of alkoxy groups into group (R2) produce effective antihistaminic compounds with reduced drowsiness.
The ethylenediamines are highly effective H1 antagonists and are useful antihistamines. They also exhibit high frequency CNS depression and gastrointestinal side effects.
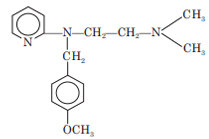
Pyrilamine
Chemistry: Pyrilamine is an ethylene diamine derivative. Pyrilamine occurs as maleate salt. Chemically it is 2-[[2- (dimethylamino) ethyl] (p-methoxybenzyl) amino] pyridine maleate.
Properties: Pyrilamine maleate is white, characteristic odor, water or alcohol soluble, crystalline powder.
Synthesis:
2-Aminopyridine by alkylation with chloroethyldimethylamine yields diamine.
Reaction of diamine with p-methoxybenzylchloride gives pyrilamine.
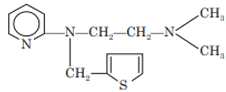
Methapyrilene
Chemistry: Methapyrilene is an ethylenediamine with pyridine nucleus. It is an H1-receptor antagonist. It is 2-[[2-(dimethylamino) ethyl] 2-thenylamino]-pyridine.
Properties: Methapyrilene occurs as hydrochloride salt. Methapyrilene hydrochloride is water soluble, white crystalline powder.
Thonzylamine
Chemistry: Thonzylamine occurs as hydrochloride salt. Chemically thonzylamine is 2-((2-(dimethylamino)ethyl) (p methoxybenzyl)amino) pyrimidine. Thonzylamine hydrochloride is a water soluble, white crystalline powder. It is an Histamine H1-receptor antagonist used for the symptomatic relief of hypersensitivity disorders.
Uses: It is recommended for use with streptomycin in exudative human tuberculosis. It is used in treating the symptoms of hay fever, urticaria, drug reactions and other mild allergic conditions.
Tripelennamine
Synthesis: Tripelennamine can be prepared as follows: 2 aminopyridine, prepared by the action of sodamide on pyridine, is reacted with β-dimethylaminoethyl chloride in the presence of sodamide, and the resulting 2-[2-(dimethylamino) ethylamino] pyridine is subsequently condensed with benzyl bromide in the presence of sodamide. The corresponding hydrochloride salt is obtained from the base by treatment with hydrogen chloride in an organic solvent.
Uses: It is frequently employed in the treatment of perennial and seasonal allergic rhinitis, allergic conjunctivitis due to inhalant allergens and foods, simple allergic skim manifestations of urticaria and angioedema, dermographism and anaphylactic reactions as an adjunct to adrenaline.
Propylamine Derivatives
The propylamine antihistamine derivatives possesses the following general structure:
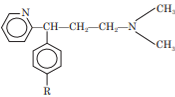
They are most active H1-antagonists and also produce less sedation.
They exhibit significant anticholinergic activity in addition to antihistaminic activity.
All propylamine antihistamine derivatives are chiral compounds.
Propylamines possesses sp3 or sp2 carbon bridge between terminal tertiary amino group and diaryl moieties.
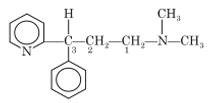
Pheniramine
Chemistry: Pheniramine is available as pheniramine maleate. It is dimethyl(3-phenyl-3-(2-pyridyl)propyl amine hydrogen maleate.
Properties: Pheniramine maleate is water soluble, white, crystalline powder.
Synthesis: Phemiramine is prepared by condensation of 2 benzylpyridine with dimethylaminoethyl chloride in presence of sodamide.
Uses: Pheniramine is an antihistaminic agent. It is effective to treat allergic rhinitis, vasomotor rhinitis, allergic conjunctivitis, mild urticaria and angioedema. It is used widely in cough preparations.
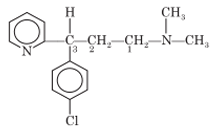
Chlorpheniramine
Chemistry: Chlorpheminamine occurs as chlorpheniramine maleate, which is an alkylamine derivative. It is (RS)-3-(4-chlorophenyl)-3-(2-pyridyl) propyldimethylamine hydrogen maleate. Chlorpheniramine maleate is water soluble, white crystalline powder.
Uses: Chlorpheniramine is a Histamine H1-receptor antagonist used for the symptomatic relief of hypersensitivity reactions including urticaria, rhinitis, conjunctivitis and angioedema. It is a potent antihistamine and causes moderate degree of sedation. It also has antimuscarinic activity.
Brompheniramine
Brompheniramine maleate is 3-(4-bromophenyl)-3-(2-pyridyl) propyldimethylamine hydrogen maleate. It is an optically active compound. Dexbrompheniramine is (+)- isomer and is more potent than (-)-isomer. It is water soluble, white crystalline powder.
Uses: Brompheniramine is an antihistamine with anticholinergic and sedative effects. It is effective for the relief of hay fever and upper respiratory allergic systems such as itchy, watery eyes, sneezing, itching nose etc.

Triprolidine
Chemistry: Triprolidine is a pyrrolidine derivative. It possesses pyrrolidine and pyridine moieties. Triprolidine occurs as hydrochloride salt. It is 2-(3-(pyrrolidin-1-yl)-1-prolylprop-1-enyl) pyridine hydrochloride.
Properties: Triprolidine hydrochloride is a water soluble, white, crystalline powder.
Uses: Triprolidine is a potent antihistamine used for the symptomatic relief of hypersensitivity reactions including urticaria, rhinitis, conjunctivitis and for pruritic skin disorders. It is also used with pseudoephedrine for the treatment of cough and common cold.
Phenothiazine Derivatives
Phenothiazines possess tricyclic system. They were introduced in 1945. General structure of phenothiazine antihistamines is:
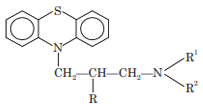
Phenothiazine derivatives possess two or three carbon chain bridge between basic phenothiazine nucleus and terminal nitrogen.
Trimeprazine
Chemistry: Trimeprazine is a phenothiazine derivative. It is 10-(3-dimethylamino2-methyl propyl)-phenothiazine. It is more active than promethazine and less active than chlorpromazine. Trimeprazine occurs as its tartarate salt. Trimeprazine is synthesized by alkylation of phenothiazine.
Properties: Trimeprazine tartarate is odorless, white colored crystalline powder. It should be protected from light.
Uses: Trimeprazine is effective for the treatment of pruritic symptoms due to urticaria. It is also effective to treat a variety of allergic and non-allergic conditions such as contact dermatitis, allergic dermatitis, drug rash etc.
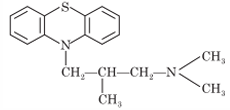
Promethazine
Chemistry: Promethazine possesses phenothiazine nucleus. Promethazine is 10-(2- dimethylaminopropyl) phenothiazine.
Properties: It occurs as hydrochloride salt. Promethazine hydrochloride is a white, colorless powder. It is freely soluble in water, soluble in alcohol, chloroform and practically insoluble in ether and acetone. It should be stored in airtight container and protect from light.
Synthesis: It is prepared by condensation of phenothiazine with 2-(N-dimethylamino)- 1-chloropropane in presence of sodamide.
Uses: Promethazine is an antihistaminic agent with some antimuscarinic, antiemetic and local anesthetic properties. It is used for treatment of allergies, anxiety prior to surgery, as an anti-nauseant, and for control of motion sickness.
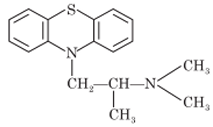
Methdilazine
Synthesis: Methdilazine is a phenothiazine derivative. Methdilazine is 10-(1-methyl-3 pyrrolidinyl)methyl)phenothiazine. It is prepared from phenothiazine by alkylation with N-methyl-3-chloromethyl pyrrolidine.
Properties: Methdilazine occurs as hydrochloride salt. Methdilazine hydrochloride is light tan crystalline powder having slight characteristic odor. It is freely soluble in water and alcohol.
Uses: It has been used as antihistaminic agent to treat seasonal and perennial allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis. It is also used to treat anaphylaxis. Methdilazine also possesses sedative effects and has local anesthetic properties.
Piperazines
Piperazines are derivatives of piperazine nuclei. The piperazine derivatives are moderately potent antihistaminics with a lower incidence of drowsiness. The general structure of piperazines is:
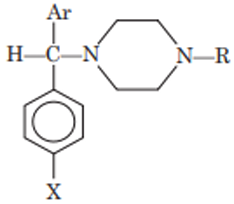
They are defined as cyclic ethylenediamines because the connecting group (CHN), the carbon chain (—CH2—CH2) and terminal tertiary nitrogen are part of piperazine moiety.
Cyclizine
Chemistry: Cyclizine is a piperazine derivative. It is 1-(diphenylmethyl)-4-methyl piperazine. Cyclizine occurs as hydrochloride salt.
Properties: Cyclizine hydrochloride available as a white crystalline powder. It is slightly soluble in water, alcohol and chloroform.
Synthesis: Cyclizine is prepared by reaction of benzhydryl chloride with N-methylpiperazine.
Uses: Cyclizine is a histamine H1-receptor antagonist given by mouth, or parenterally to control post-operative and drug induced vomiting, and motion-sickness.
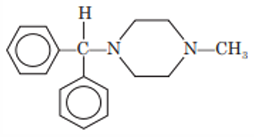
Chlorcyclizine
Chemistry: Chlorcyclizine is also a piperazine derivative. It is 1- (p-chlorobenzhydryl)- 4-methylpiperazine. The antihistaminic activity of piperazines is reduced if halogen is introduced at 2 or 3 position of phenylring ring structures.
Synthesis: Chlorcyclizine is prepared by condensation of p- chlorobenzhydryl chloride with N-methylpiperazine.
Uses: Chlorcyclizine has antihistaminic activity. It is used to treat urticaria, hay fever and other allergic conditions.
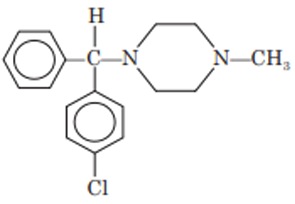
Meclizine
Chemistry: Meclizine is a piperazine derivative. Meclizine occurs as hydrochloride salt. It is 1-(p-chlorobenzhydryl)-4-(m- methylbenzyl) piperazine.
Properties: Meclizine hydrochloride is water-insoluble, tasteless, white or slightly yellowish crystalline powder.
Synthesis: It is synthesized by alkylation of p-chloro analog of monosubstituted piperazine with m-methylbenzyl chloride.
Uses: Meclizine has moderate antihistaminic activity. It is also used to treat nausea and vomiting.
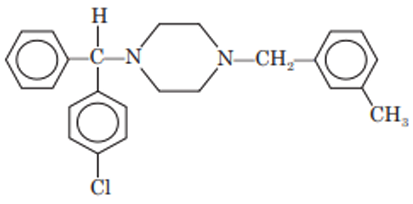
Buclizine
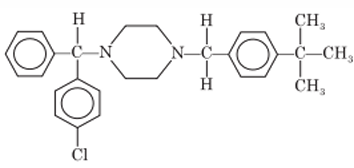
Chemistry: Buclizine is another piperazine derivative. It is 1-(p-tert butylbenzyl)-4- (p-chloro-α-phenylbenzyl) piperazine. Buclizine occurs as hydrochloride salt.
Properties: Buclizine hydrochloride is white or slightly yellow colored, crystalline, water insoluble powder.
Synthesis: Condensation of p-chloro analog of monosubstituted piperazine with p-tertiary butylbenzyl chloride gives buclizine.
Uses: Buclizine has antihistaminic and antiemetic properties.
Dibenzocycloheptenes
Dibenzocycloheptenes possess H1 antihistaminic activity. Dibenzocycloheptenes are phenothiazine analogues. The sulfur atom of phenothiazine nucleus is replaced by an isosteric vinyl group (—CH=CH—) or a saturated ethyl bridge (—CH2—CH2). A sp2 hybridized carbon atom replaces the nitrogen of phenothiazine.
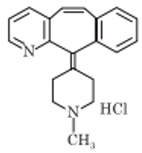
Cyproheptadine
Cyproheptadine is a histamine H1-receptor and serotonin antagonist. It occurs as hydrochloride salt. Chemically it is 4- (5H-dibenzo[a,b]cyclohepten-5-ylidene)-1-methylpiperidine. Cyproheptadine hydrochloride is white to slightly yellow, odorless, crystalline powder.
Synthesis:
Ketone is brominated with N-bromosuccinimide, followed by dibrominated with triethylamine to get an alkene.
Alkene reacts with N-methyl-4-piperidyl magnesium chloride (Grignard reagent) to yield a complex which is treated with HCl to get cyproheptadine HCl.
Uses: Cyproheptadine is used for the symptomatic relief of hypersensitivity reactions and in pruritic skin disorders. It is also used to treat migraine, anorexia and diarrhea of carcinoid syndrome.
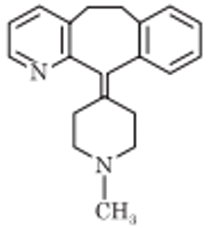
Azatadine
Chemistry: Azatadine is chemically related to cyproheptadine. It differs from cyproheptadine in having of pyridine ring instead of benzene ring. Azatadine is white crystalline water-soluble powder occurs as maleate salt. Chemically azatadine is 6,11 dihydro-11-(1-methyl4-piperidylidene)-5H-benzo(5,6)-cycloheptal (1, 2-b) pyridine.
Miscellaneous Antihistamines
Phenindamine
Chemistry: Phenindamine occurs as tartarate salt. Chemically it is 2,3,4,9-tetrahydro-2-methyl-9-phenyl-1H-indeno [2,1-c] pyridine.
Properties: Phenindamine tartarate is odorless, creamy white, water-soluble powder. The solutions of phenindamine tartarate are slightly acidic to litmus.

Dimethindene
Chemistry: Dimethindene is an indene derivative occurs as maleate. Chemically, it is 2-[1-[2-[2-(dimethylamino)ethyl]inden-3-yl] ethyl] pyridine. Dimethindene is synthesized by adding 1-(2-pyridyl)ethyl lithium to 2-[2-(dimethyl amino)ethyl] indan-1-one.
Properties: Dimethindene maleate is white, characteristic odor, crystalline powder. It is freely soluble in methanol, chloroform but slightly soluble in water and is sensitive to light.
Uses: It is a potent antihistaminic agent used in perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis.
Antazoline
Chemistry: Antazoline is an imidazoline derivative. Antazoline is 2-[(N-benzylanilino) methyl]-2-imidazoline. It is synthesized by alkylation of benzylaniline with halogenated imidazoline.
Properties: It occurs as phosphate salt. Antazoline phosphate is white, bitter taste, crystalline powder. It is soluble in water.
Uses: Antazoline is histamine H1-receptor antagonist used for the treatment of rhinitis and conjunctivitis.
Ebastine
Ebastine is a potent, long acting antihistaminie. It acts against both early and late phase without any adverse effect on the cardiovascular and central nervous system. Ebastine is a piperidine derivative. Chemically ebastine is 4-tertiarybutyl 1-4(4-diphenyl methoxy piperidine) butyrophenone.
Mechanism of Action: Ebastine and its active metabolite carebastine, are selective histamine H1 peripheral receptor antagonists devoid of untoward CNS action and anticholinergic effects. Because of low lipophilicity and greater molecular size it has limited ability to cross the blood brain narrower, allowing an effective blockage of H1 receptors in peripheral issues without important central side effects.
Uses: Allergic rhinitis (seasonal and perennial). Idiopathic chronic urticaria.
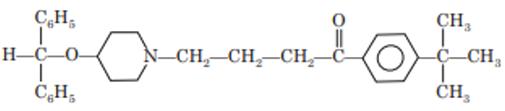
Therapeutic Applications
All antihistamines are of potential value in the treatment of nasal allergies, particularly seasonal allergic rhinitis (hay fever), and they may be of some value in vasomotor rhinitis. They reduce rhinorrhoea and sneezing but are usually less effective for nasal congestion. Antihistamines are used topically in the eye, in the nose, and on the skin.
Oral antihistamines are also of some value in preventing urticaria and are used to treat urticarial rashes, pruritus, and insect bites and stings; they are also used in drug allergies. Injections of chlorpheniramine or promethazine are used as an adjunct to adrenaline in the emergency treatment of anaphylaxis and angioedema. Some antihistamines (including cinnarizine, cyclizine, and promethazine teoclate) are used to control nausea and vomiting. Buclizine is included as an antiemetic in a preparation for migraine.
Second Generation Antihistamines
The second-generation antihistamines bind only to peripheral H1 receptors, and reduce allergic response with little or no sedation. They have no central action, and are used only for treatment of allergic reactions. These drugs have prolonged antihistaminic effects and also binds to muscarinic, and adrenergic receptors. These are divided into two chemical classes
Piperazine Derivative

Piperidine Derivatives
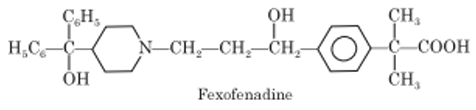
Histamine H2- Receptor Blockers
Some of the histamine responses (dilation of capillaries, increasing the rate and force of heart contraction, increasing gastric acid secretion) are exclusively mediated through H2 receptors. The classical antihistamines are unable to inhibit these responses hence H2 receptor-blocking drugs were introduced. H2-Receptor antagonists are drugs used to block histamine H2 receptors reversibly and inhibit H2-receptor mediated responses. They are used to treat gastric ulcers as they block gastric-acid and enzyme secretion. Gastric acid secretion by parietal (oxyntic) cell is not blocked by antihistamines acting on H1 receptors. They have little affinity for H1-receptors.
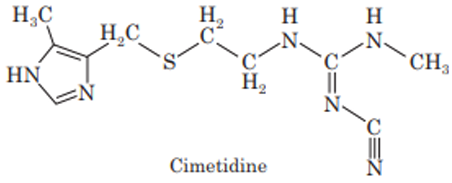
Cimetidine
Cimetidine is a H2-histamine receptor antagonist. It inhibits basal as well as meal stimulated gastric secretion. Cimetidine also inhibits cytochrome P450. Cimetidine acts on H2-receptors in the stomach, blood vessels and thus inhibits gastric acid secretion. It is used in the treatment of ulcers such as duodenal ulcer, non-malignant ulcer etc.
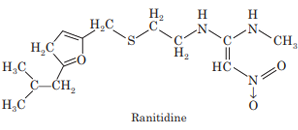
Ranitidine
Ranitidine is another H2-histamine receptor antagonist. It is 5- 10 times more potent than cimetidine. Ranitidine was developed because it binds less to cytochrome P450 and has fewer side effects and acts longer.
Famotidine
Imidazole moiety was replaced by thiazole to get famotidine. Famotidine is a newer H2-histamine receptor antagonist. It is more effective than ranitidine, less than 45% of an oral dose is absorbed.

Side Effects
Central nervous system reactions include drowsiness, sedation, dizziness , faintness, disturbed coordination, lassitude, confusion, restlessness, excitation, tremor, seizures, headache, insomnia , euphoria, blurred vision, hallucinations , disorientation, disturbing dreams/nightmares, schizophrenic like reactions, weakness, vertigo, hysteria , nerve pain, and convulsions. Overdoses may cause involuntary movements.
Gastrointestinal problems include increased appetite, decreased appetite, nausea, vomiting, diarrhea.
Hematologic reactions are rare, but may be severe. These include anemia, or breakdown of red blood cells; reduced platelets; reduced white cells; and bone marrow failure.
A large number of additional reactions have been reported. Not all apply to every drug, and some reactions may not be drug related. Some of the other adverse effects are chest tightness; wheezing ; nasal stuffiness; dry mouth, nose and throat; sore throat ; respiratory depression; sneezing; and a burning sensation in the nose.