Chapter 7_Particular Nature of Matter_G3
The Particulate Nature of Matter
Page 1: Introduction
Particulate Nature of Matter: A model use to represent and explain matter (e.g: pollen grain in water)
The particular Nature of Matter states that:
All matter is made up of small, discrete particles
All particles of one substance are identical
Particles of all matter are in constan and random motion
Thus this model it used to explain the properties of different states of matter
Page 11-12: States of Matter
Questions to Consider:
Why do solids have fixed shapes?
Why do liquids take the shape of their containers?
Why are gases compressible?
Page 13-15: Particulate Models
Solid:
Movement: Particles can only vibrate about in their fixed positions
Arrangement:
Very strong forces of attraction between partices
Particles are packed closely together in a regular pattern
Properties:
Has a definite shape and volume
Occupies a smaller volume than liquids and gases
Has a higher density than liquids and gases
Cannot be compressed
Liquid:
Movement: Particles can slide over one another.
Arrangement:
Strong forces of attraction between particles
Paricles are packed closely together in an irregular pattern
Properties:
Has no definte shape
Has a fixed volume
Occupies more volume than solids
Has lower density than solids
Cannot be compressed
Gas:
Movement: Particles can move at high speed in all directions
Arrangement:
Weak forces of attraction between particles
Particles are very far apart and randomly arranged
Properties:
Has no defnite shape or volume
Occupies the largest volume
Has the lowest density
Can be compressed
Why do solids have a fixed shape?
Are held together by very strong forces of attraction
Vibrate about in their fixed positions
Cannot move about freely
Why do liquids take the shape of the containers they are in?
Are arranged in a disorderly manner
Have weaker forces of attraction
Are not held in fixed positions
Can move freely throughout the liquid
Why are gases compressible?
Particles in gas are far apart from one another. There is space for the particles to be compressed.
Why do gases have a lower density than solids?
In a closed container, when a solid turns into gas
The mass of the substance remains the same
The volume increases
Since Density= Mass/Volume, the density od the substance decrease
How do the movement and arrangement of particles change when a solid turns into a liquid
FIrst state arrangement/movement must be stated. Then can u state about when the first state into the second state
Arrangement: In solid, the particles are very vlosely packed together in an orderly manner. When the solid turns into liquid, the particles are still closely packed together but in an disorderly manner (FIrst state arrangement must be stated. Then can u state about when the first state into the second state.)
Movement: In Solid, the particles vibrate about in their fixed position. When the solids turns into a liquid, the particles are able to slide over each othedr freely.
Page 22-28: Expansion and Contraction
Heat Effects:
When matter gains or loses heat, the particles will change in their movement and arrangement
Expansion: Gaining heat increases particle movement, causing volume to increase.
Contraction: Losing heat decreases particle movement, causing volume to decrease.
Mass Consistency: Mass remains the same during expansion and contraction.
What happens during expansion
When matter is heated, the particles gain energy and vibrate more vigorously
The particles move slightly futher apart from one another, causing the vloume of the matter to increase. The process is called expansion
What happpens during contraction
Then matter is cooled, the particles lose energy and vibrate less vigorously
The particles move closer to one another. This causes the volume of matter to decrease. This process is called contraction
Why does the mass remain the same
During expansion an contraction, the distance between the particles of the metal lock changes
Although the volume of the matter changes, the size and amount of the particles of matter do not change
Page 31-47: Changes in States
Melting - solid to liquid
When a solid is heated, the particles gain energy and vibrate more vigorously
When the particles gain enough energy, they overcome the strong forces of attraction between one another. The particles break free from one another and move randomly.
Melting occurs when the melting point is reached. The solid changes to its liquid state
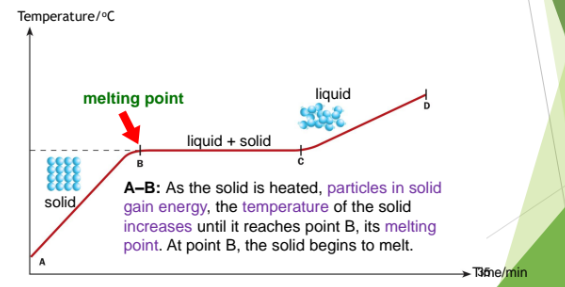
From A to B: As the solid is heated, the particles in the solid gains energy, the temperature of the solid increases until it reaches point B, its melting point. At point B, the solid begins to melt.
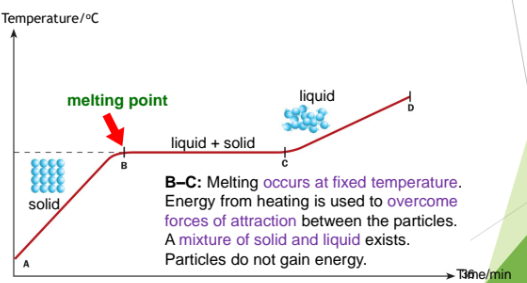
From B to C: Melting occurs at fixed temperature. Energy from heating is used to overcome forces of attraction between the particles. A mixture of solid and liquid exists. The particles so not gain energy.
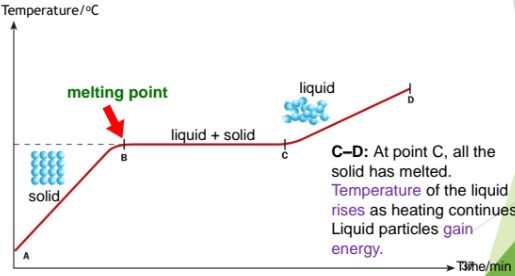
From C to D: At point C, all the solid has melted. The temperature of the liquid rises as the heating continues. Liquid particles gain energy
Freezing - liquid to solid
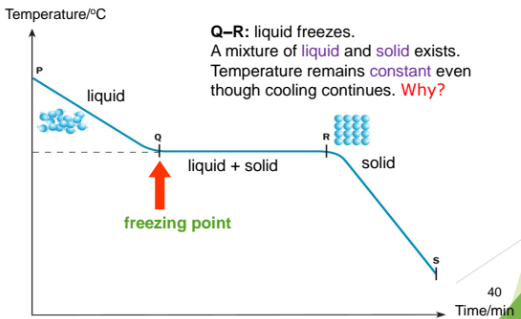
When a liquid is cooled, the particles lose energy an move musch slower
When the particles lose enonugh energy, they cannot overcome the forces of attraction between one another and move less randomly
At this point, the particles return to their fixed positions. The liquids changes to its solid state

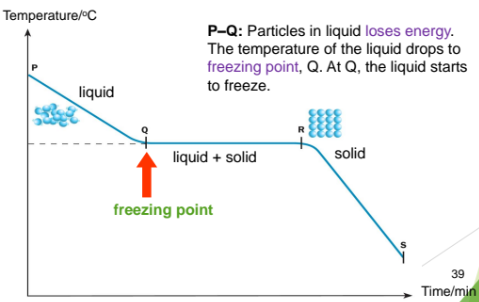
From P to Q: Particles in the liquid loses energy. The temperature of the liquid drops to freezing point, Q. At Q, the liquid starts to freeze
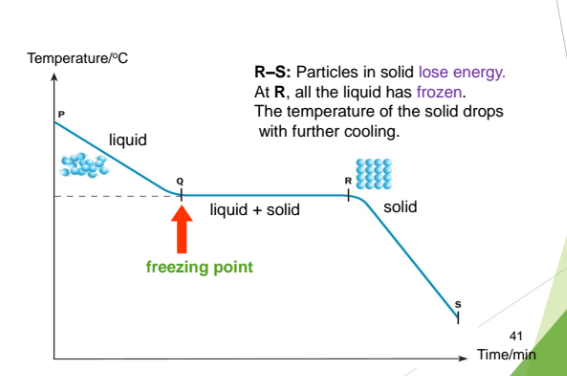
From R to S: Particles in solid lose energy.
At R, all the liquid has frozen.
The temperature of the solid drops
with further cooling.
Boiling - liquid to Gas
When a liquid is heated, the particles gain energy and vibrate more
When the particles gain enough energy, they overcome the strong forces of attraction between one another. The particles break free from one another and move randomly. E.g. When liquid water is heated up to 100°C, it boils to become steam

Boiling occurs when the boiling point is reached. The liquid changes to its gas state.
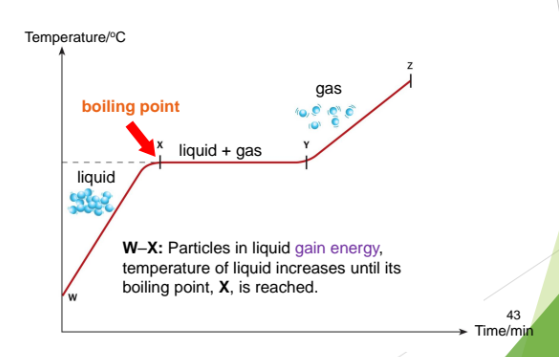
W-X: Particles in liquid gain energy, temperature of liquid increases until its boiling point, X, is reached
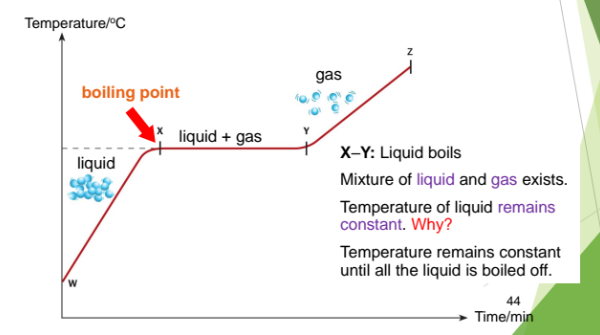
X–Y: Liquid boils
Mixture of liquid and gas exists.
Temperature of liquid remains constant. Why?
Temperature remains constant until all the liquid is boiled off.
Condensing – gas to liquid
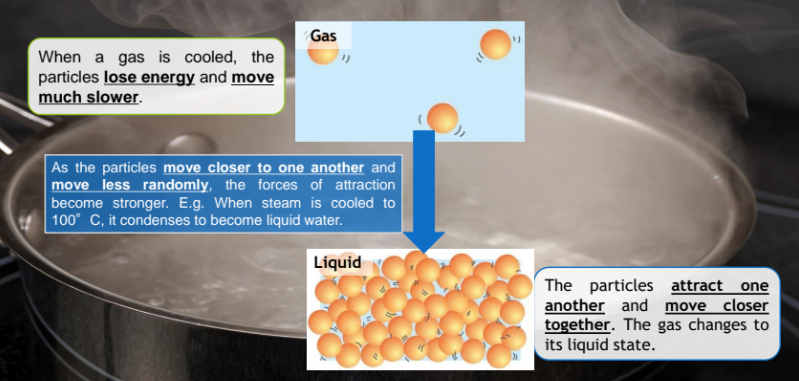
When a gas is cooled, the particles lose energy and move much slower
As the particles move closer to one another and move less randomly, the forces of attraction become stronger. E.g. When steam is cooled 100°C, it condenses to become liquid water.
The particles attract one another and move closer together. The gas changes to its liquid state.
Summary
During melting and boiling…
Heat is gained
to overcome the forces of attraction between the particles,
allowing the particles to break free from one another and move more randomly
During freezing and condensing…
Heat is lost,
resulting in stronger forces of attraction,
and hence, particles move closer to one another and move less randomly.
Checkpoint 5
Using the Particulate Model of Matter, describe how a solid becomes a liquid.
A solid becomes a liquid due to a gain in heat.
As the heat increases, the particles gain enough energy to overcome the forces of attraction between one another, allowing them to move more randomly and further away from each other.
Diffusion
Think of the last time you detected the smell of perfume in a room. How has the smell travelled to your nose?
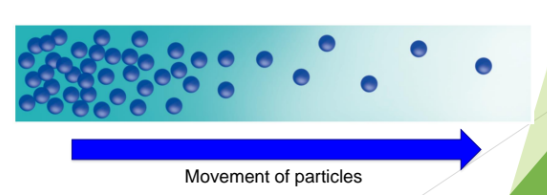
What is a concentration gradient and how is it related to diffusion?
The particles are in constant and random motion
Particles will diffuse down their concentration gradient.
Diffusion is the net movement of particles from a region where they are of higher concentration to a region where they are of lower concentration, that is, down a concentration gradient
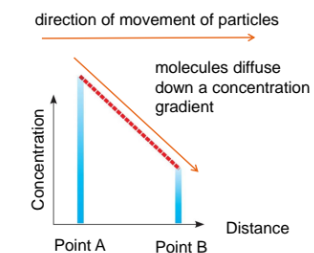
The concentration gradient is the difference in concentration between two regions.
The steeper the concentration gradient, the faster the rate of diffusion
How can we show diffusion of a dissolved substance?
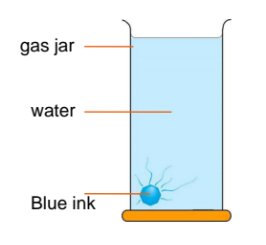
Drop a blue ink into a gas jar containing water.
Allow it to stand for a few days.
Explain why the blue colour gradually spreads throughout the water.

There is high concentration of ink particles at the bottom of the water.
The ink particles move from a higher concentration at the bottom to the lower concentration in other parts of the water randomly.
Eventually the water becomes uniformly blue due to
the overall or net movement of the ink particles.
Diffusion-weighted magnetic resonance imaging (DWRI)
Diffusion-weighted magnetic resonance imaging (DWI or DW-MRI) is the use of specific MRI sequences as well as software that generates images from the resulting data that uses the diffusion of water molecules to generate contrast in MR images
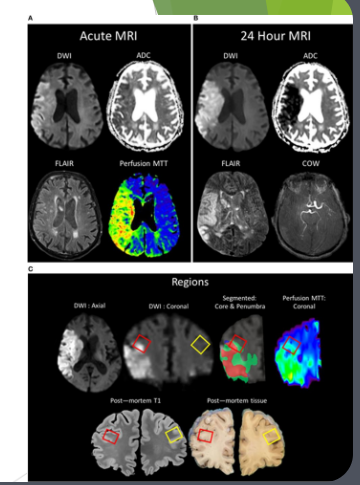
When do we use DWRI
Look at brain activity in stroke patients
Check for cancer tumours
Page 49-56: Diffusion
Definition: Movement of particles from high to low concentration.
Concentration Gradient: Difference in concentration drives diffusion; steeper gradients lead to faster diffusion.
Example: Ink spreading in water due to diffusion.
Application: Diffusion-weighted MRI used in medical imaging to assess brain activity and tumors.
Page 57: Practice and Activity
Practice Questions: Engage with material to reinforce understanding.
This note summarizes the key concepts and details from the transcript regarding the particulate nature of matter, its states