Mid-Term Exam Review
Lesson 1 – Average Atomic Mass
Mass Number = Atomic Number (Represents number of Protons) + number of neutrons
Abundance of Each Isotope
o u = Mass 1*Abundance 1(in decimal) + Mass 2*Abundance 2 …
o Avgamu = m1p1+m2p2…
o Must write out for full communication Marks.
Must multiple the relative abundances of the element’s isotopes by their atomic masses then summing the products
the average atomic mass on the periodic table is the average of all the masses of all the isotopes
Isotopes
o Most abundant when the -n number is closes to the one on the periodic table.
o To find the abundance from the mass must use:
o Uses the thing on the periodic table form Avg amu.
o There are 2 equations one is always about p1+p2=1 as they must equal 100%
o Rearrange for the x and substitute.
Lesson 2 – The Quantum Model
“n”
o Whole number starting at 1 only up to 7.
o Goes 1=2, 2=8, 3=18
o Electrons, waves, and particles
o Never in a fixed position
o There is probability density of where the electrons could be at that moment but is always moving around (ELECTRON DENSITY) → Never actually know where an electron is at any given moment.
o Schrödinger created this and made ATOMIC ORBITALS
Atomic Orbital Shapes
o S = Spheres = 2 electrons (1 Orbital)
o P = dumbbells = 6 electrons (3 Orbitals)
o D = flower-like = 10 electrons (5 Orbitals)
o F = complicated flowers = 14 electrons (7 Orbitals)
Rules of Filling
o Hund’s
o Half fill first then full fill
o Pauli’s Exclusion Principle
o No electron can have same set of 4 quantum numbers (ONE SPINS ONE WAY AND THE OTHER SPINS THE OTHER WAY)
o Aufbau Principle
o When doing the filling must make sure that after 56 (Ba) must go to the 57 or La so go down and that starts at 4f and then only has also 5s
Polar VS None Polar Electronegativity
under 0.5 → non-polar covalent
0.5-1.6 → polar covalent
1.6-2.0 → polar covalent if 2 non-metals or ionic is 1 non-metal and 1 metal
over 2 → Ionic
could also be angles with VSEPR (already in note with the bond angles)
Periodic Trends
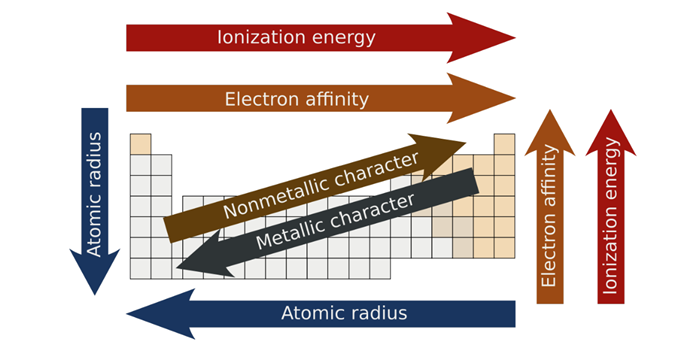
o Periodic Law = Something that repeats and follows a pattern
Effective Nuclear Charge
o How strong the attraction is between the positive nucleus and the negative outer electrons.
o As you increase the number of protons you increase the force of attraction
o As you increase the number of inner electrons you decrease the force of attraction using the SHEILDING EFFECT
Atomic Radius
o The size of the NEUTRAL ATOM
Ionic Radius
o The size of an ION (IN COMPARISON TO THE NEURTAL ATOM OR OTHER ATOMS)
o Cation: become smaller as loses electrons POSITIVE CHARGE
o Anion: become bigger as there is an addition of electrons NEGATIVE CHARGE
Ionization Energy
o How much energy it takes to remove ONE VALENCE ELECTRON
o More energy = higher ionization energy
o Atom(g) + energy = ion++ e-
Electron Affinity
o Opposite of Ionization Electron
o How much energy RELEASED with one electron is added.
o Must be released to be able to add the electron to decrease the stress on the atom.
o This is a NEGATIVE number.
o Greater negative = great want for an electron
o i.e. Florine has the highest electron affinity, so they are wanting the electron the most.
Electronegativity
o An atom’s tendency and want to attract the electron shared in a bond or strip in an ionic compound of its valence electrons.
Lesson 4 – Bonding and Structures
Structures of Ionic Bonds and Properties
o Crystal LATTICE STRUCTURES
o High boiling and melting point (Strong Bonds)
o Conducts electricity when dissolved in water (IONS)
o Conducts electricity as molten liquids but not as a solid (DOES NOT MOVE SO CAN’T CONDUCT)
o Hard and brittle (repulsion between charges)
Structure of Molecule and Properties
o Low melting and boiling point (weaking bonds)
o Poor conductivity (Neutral no ions)
o Solid, liquid, gas at Room temperature (Depends on the intermolecular forces involved)
o Usually soft and waxy, flexible (BONDS ARE FLEXIBLE)
o Can be crystalline but that is an exception.
o Electronegativity
o LINUS PAULING FOR IMPORTANT PROPOSION
o Less than .5 – nonpolar
o .5-1.6 -polar
1.7-1.9 - FIGURE OUT WHAT IT IS MADE OF
o More then 2 – ionic
Lesson 5 – Molecule Polarity
Dipoles
o This is a vector.
o is created when their ware regions of negative and Positive charge.
o NOT FORMAL CHARGE but when added all together can find formal charge, must have a full symmetry of all outside elements in the molecule to POSSIBLY either a polar or non-polar element.
Shapes of Molecules with Molecule Polarity
o Electron Groups – one double, single, or triple bond or lone pair
o Electron Geometry – arrangement of electrons (EG) → number of electron groups
o Molecular Geometry – shape of molecule (ONLY LOOK AT THE CENTER OF THE ATOM) -→ how they are arranged
Family of 2 – Linear
o Has Two Electron Groups
o They are as repelled as possible, so they are balances.
o Non-polar as dipoles cancel each other out, if the same element
Polar if the elements are of different electron negativity
Family of 3 – Trigonal Planar
o Has 3 electron Groups
o If symmetrical then non-polar
o Some middle elements are happy with 6 electrons
Family of 4
Tetrahedral, Tetrahedral
o 4 electron groups (4 bonds)
o One forward, one left, one right, one back
o Non-polar, unless onto all the same then polar
Tetrahedral, Trigonal Pyramidal
o 4 electron groups, one lone pair, 3 bonds
o Polar
o Side, forward, back
o 109.5
Tetrahedral, Bent
o 4 electron Groups, 2 orbitals, 2 bonds
o Polar
o one left, one right, orbitals on top
o WATER: BOND ANGLE IS 104.5
o NORMAL: 109.5
Lesson 6 – Intermolecular Forces (IMFs)
Attraction that keeps molecules together, stronger forces the closer the molecules.
Properties of Compounds
o Melting Point: Solid to liquid
o Boiling Point: liquid to gas
o Solubility: can it dissolve into water (LIKE DISSOLVES LIKE) (POLAR DISSOLVES POLAR)
o Conductivity: Allows electric Current (Only with Ions)
o Vapour Pressure or Volatility: balance between particles in that has and water phases. Higher volatility is lower boiling point.
London Dispersion Force (LDF)
o ALL COMPOUNDS HAVE THIS
o This is when there is a temporary dipole as the electrons are always moving for cloud to cloud.
o This attracts each other in a compound.
o Typically, the weakest IMF unless it has an extremely high molecular mass which would lead to a high LDF.
o Molecular mass directly affects LDF as they are more loosely held, also this would increase the surface area which give more space for the electrons to hop around.
Dipole-Dipole Force
o Always have a permanent dipole with polar molecules
o Electrons are always being pulled in one direct or another so there will always be a charged side that will be attracted to it à forms a link of SAME COMPOUND TOGETHER
o Like electrostatic forces between ions but you don’t have fully charged atoms, just an uneven electron sharing.
Hydrogen Bonding
o Special type of dipole force between hydrogen and Nitrogen, Oxygen, or Florine
o Hydrogen is strongly negatively charge; this is the strongest IMF.
When there is an addition of molecular mass, dipole-dipole, or hydrogen bonding the melting and boiling point will increase as the forces become stronger.
Organic Naming
Prefix | # of Carbons | Functional Group | Suffixes | General formulas |
Meth- | 1 | Alkanes – single | -ane | CnH2n+2 |
Eth- | 2 | Alkenes -double | -ene | CnH2n |
Prop- | 3 | Alkynes – triple | -yne | CnH2n-2 |
But- | 4 | Alcohols – OH | -ol | CnH2n+2OH |
Pent- | 5 | Carboxylic Acids – COOH at end | -oic acid | CnH2n+1 COOH |
Hex- | 6 | Ring | Cyclo- |
|
Hept- | 7 | Benzene | Six with 3 double bonds |
|
Oct- | 8 | Make sure that there are numbers | Indicating what they are bonded too |
|
Non- | 9 | NEED TO MAKE SURE | Least to most |
|
Dec- | 10 | Can be at start or at the middle | Commas need between numbers |
|
Unit 2 Reactions
KNOW SYNTHESIS, DECOMPOSTION, SINGLE DISPLACEMENT, DOUBLE DISPLACEMENT
Combustion Reactions
o CnHm + O2 → CO2(g) + H2O(g) → Complete
o CnHm + O2 → CO2(g) + H2O(g) + C(s) + CO(g)
o Aqueous solutions dissolved in water à the molecule will disassociate want to become the ions.
Neutralization Reactions
o Acid (H+) + Base (OH-) → Ionic salt + water (LIQUID THAT COULD BE THE PRODUCE FOR NET IONIC EQUATIONS)
o Can also be a gas and make CO2(g) and H2O(l) Which is with sulfides and Carbonates
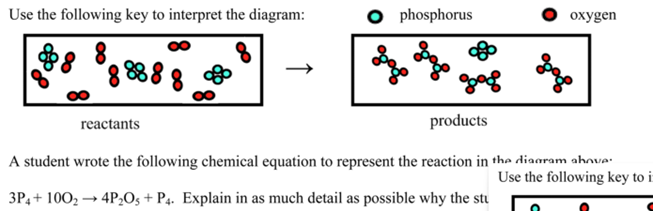
Particulate Diagrams
o Show how particles are behaving in solutions.
o Circles are one particle and are annotated to become molecules.
FOR AQUEOUS ALL PARTS MUST BE SEPERATED YET AROUND ONE ANOTHER
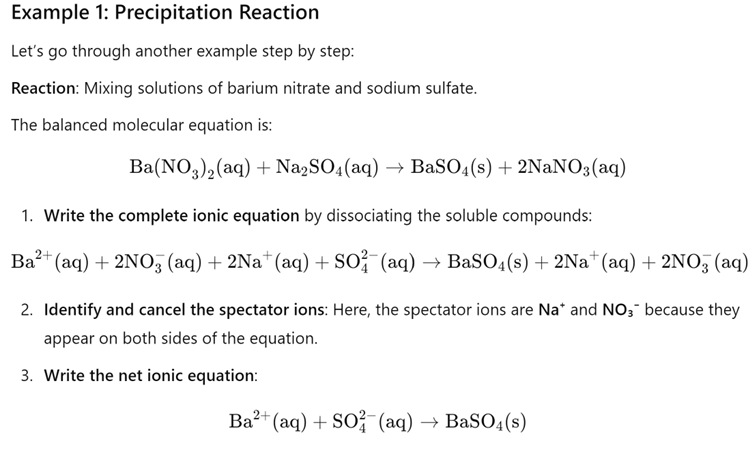
Net Ionic Equations
1. Balanced chemical equations for the reaction.
2. Write out total ionic equation à all subscripts become number in the front à must add charge.
a. Leave the solid compound as is à DON’T SPLIT IT
3. Cancel out any ions that do not appear in the solid compound à THEY STAY THE SAME STATE
4. Rewrite final net ionic equation à WITH IONS THAT MAKE THE SOLID
Example with steps:
Redox
o Oxidation = loss of election = reducing agent
o Reduction = gain of election = oxidizing agent
Oxidation number
o Changes of the atom would have if electrons were not started by transferred.
o SIGN BEFORE
o RULES FOR OXIDATION NUMBERS
o Elemental form: N.O. = 0
o Monoatomic form: N.O. = ionic charge
o Sum of N.O. values for a compound in a molecule =0
o Polyatomic: N.O. total = ions charge
Common/specific values for groups
o Oxygen: -2, unless peroxide (YxOx) then oxygen is a charge of -1
o Hydrogen: +1 when nonmetals, -1 with metals
o Group 17: -1 in combinations with metals and nonmetals (WITH OXYGEN THE HALOGEN TAKES THE + UNLESS ITS FLORINE THE -)
o Group 2: +2 in all compounds
o Group 1: +1 in all compounds
Reactions
1. Assign all oxidation numbers in elements.
2. Determine if any changes in oxidation numbers have occurred.
a. Increase or decrease.
b. Use the chart!!!
3. BOTH INCREASE AND DECREASE NEED TO HAPPEN IF A REDOX HAPPENS
o Half reactions
o Oxidized is on the right.
o Reduced is on the left.
o When calculating how many electrons you are working with, they must equal each other in both the reduction and oxidation.
o The electron you are working with is equal to the coefficient multiply by the coefficient multiplied by the oxidation number.
o When writing keep the subscripts and the coefficients in the normal place and do not multiple
Nuclear Reactions
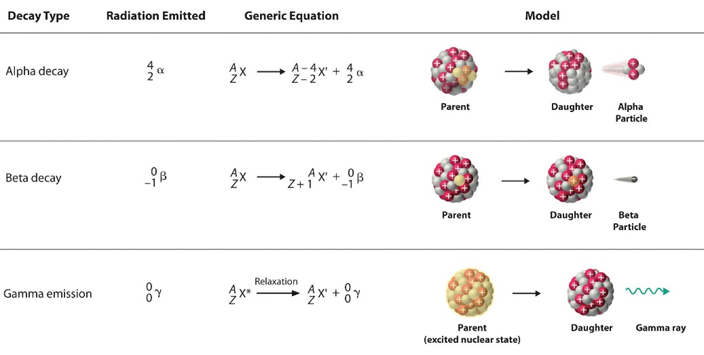
Nuclear Half Life
o The time it takes for ½ of the quantity of the substance to decay.
o The radioactive isotopes emit radiation, so they decay themselves.
o TO CALCUATE: AF = AI(½)t/p à just use Log to find the t or p
o Uses in reality: x-rays, carbon dating (C-14) , to kill cancer with radiation,
Nuclear Reactions
o Fusion: lighter nuclei combine into a heavier one
o Energy is released.
o The sun
o Fission: heavier nucleus splits into lighter ones
o Energy is released.
o Nuclear power plants and atomic bombs
Types:
ATFER | ON RIGHT | BEFORE | ON LEFT |
Proton emission | 1+1p or (H) | Proton absorption | 1+1p or (H) |
Electron emission | 0-1e | Electron absorption | 0-1e |
Positron emission | 0+1e | Positron absorption | 0+1e |
Neutron emission | 10n | Neutron absorption | 10n |
Unit 3 - Stichometry
Significant Digits
non-zeros are significant
Sandwich zeros and trailing zeros AFTER A DECIMAL are significant
leading is NEVER significant
Multiplication and Division
just least number of significant figures
Addition and Subtraction
Least number of decimal places (if no decimal then just place value/# of sig figs)
Mixed
Use BEDMAS
Round everything then use it on the calculator but write rounded
Molar Relationship
Just g/mol-1 so the number of the element times the molecule mass
Percent Composition
Tells you how much of the mass is that element in percent
Formula: MEMORIZE
% of Element = Molar Mass (Periodic Table, in g*mol-1) * # of atoms of element in chemical Formula DIVIDED BY the Molar mass of the compound (The # of Molar masses added)
Empirical Formula
Simplified/most reduced ratio → proportions of each element in a compound
Steps:
Assume you have 100g (so 1% = 1g) of the sample (if not give mass in grams, then just use that)
Covert % of element to mass (grams)
Convert Mass to Moles
Do this by multiple the full number by the reciprocal of the period table number 1/x or just divide by the mass (in g/mol)
Determine the WHOLE-NUMBER ratio of moles by dividing by the smallest number of moles (smallest of the number of element you have) → if not in whole numbers just multiple by the denominator of the fraction its in BY ALL OF THEM TO STILL GET THE RATIO
Then just write the ratio in a therefore statement
Molecular Formula from Empirical Formula
The actual ration of elements in a given substance
USE THE STEPS ABOVE TO FIND THE EMPRICAL FORMULA THEM USES FORMULA BELOW TO FIND THE FINAL
NEED TO KNOW Empirical formula and molar mass
Formula: MEMORIZE
Molecular Formula Mass DIVIDED BY Empirical Formula = RATIO (what you have to multiple by each subscript to get the formula)
Empirical Formula → the Mass on the period table multiplied by the number in each \
MUST SHOW ALL OF THE MUTIPLCATION F THE RATIO AND THE FORMULA