3.8 Representation of Solutions
Ionic
|
|
|---|---|
Soluble Ionic (NaCl) in Water | Insoluble Ionic (KCl) in Water |
Covalent
Molecule stays bonded (OF2), water molecules will be attracted based on dipoles | Molecule stays bonded (CCl4) and will form temporary dipoles based on how water molecules arrange |
|---|---|
Polar Covalent (OF2) in Water | Nonpolar Covalent (CCl4) in Water |
Hydrogen Bonding
|
|---|
Hydrogen Bonding (NH3) in Water |
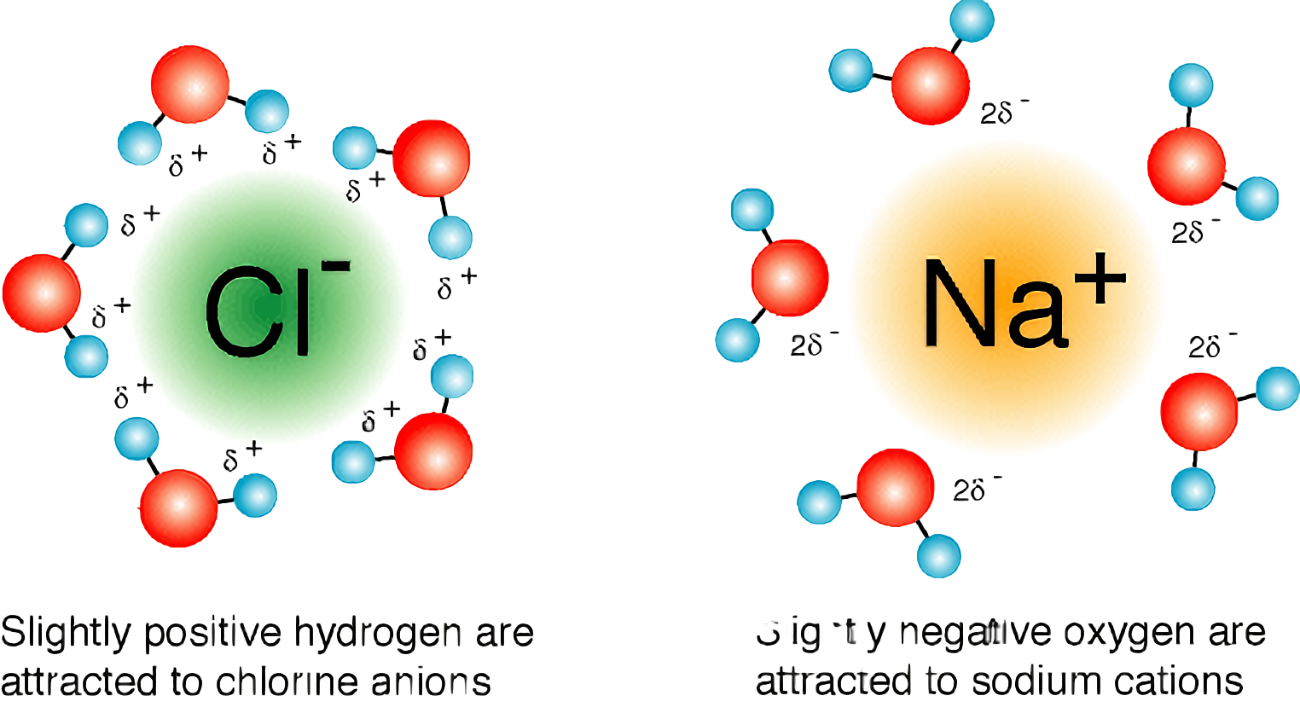 Ions will separate
Ions will separate  Some ions will dissolve, most will remain clumped at bottom
Some ions will dissolve, most will remain clumped at bottom