Chapter 15: Microbial Pathogenicity
Virulence: Allows pathogen to cause disease
Virulence genes:
Chromosomal based
plasmid or phage based
Virulence factors:
Adherence
Cell invasion
Immune response inhibitors
Colonizations
Toxins
Primary vs. opportunistic pathogens
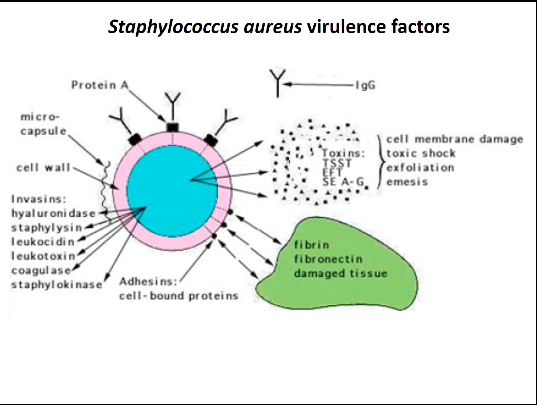
Staphylococcus aureus virulence factors
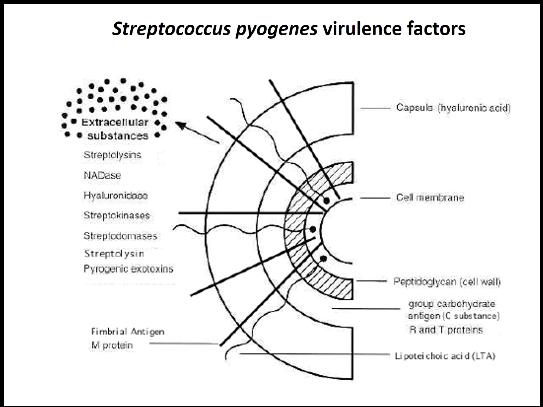
Streptococcus pyogenes virulence factors
Pathogen → Host: The Process of Infection & Causing Disease
Enter Host
Portals of Entry
Mucous membrane - respiratory tract; gastro-intestinal (GI) tract; genitourinary tract
Skin: hair follicles, sweat glands, conjunctiva
Parenteral route: deposition of microbes directly under skin or mucous membrane
Pathogens have preferred portals of entry. Entering through a different portal may present with milder or no symptoms at all
Also need to consider adhesion and number of infecting microbes
Penetrate/Evade host defenses
Capsule
Enzymes
Fimbriae/Pili
Damage host
Toxins
intracellular pathogens
Exit host
Portal of entry is generally the same portal for exit
Infectious dose - number of infectious agents required to cause disease symptoms
Measure of virulence
ID50 - infectious dose needed to cause disease symptoms in 50% of experimental hosts
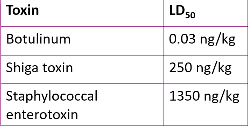
Lethal dose - number of pathogens required to kill host
LD50 - dose of pathogen required to kill 50% of experimental group of animal hosts
measure of potency
Attachment of pathogens at portal of entry
Bind via adhesins/ligands on the pathogen to host cell receptors
Examples: glycocalyx, fimbriae, M protein of Streptococcus pyogenes
Biofile formation - microbial community contained in an exopolysaccharide matrix; adhere to surfaces
Very resistant
dental plaque, catheters, IV’s, heart valves
Penetration of Host Defenses
Factors that allow for bacterial invasions of host:
Capsule - impairs phagocytosis by host cells
Cell wall components:
cell wall mycolic acids of Mycobacterium tuberculosi - has thick hydrophobic envelope which can serve as a way to avoid the immune system
M protein: Streptococcus pneumoniae
aids in attachment
anti-phagocytic properties
inactive complement
OPA protein of Neisseria gonorrhea & other neisseria
aids in attachment
Transcytosis - transport through cell layers
Allows the pathogen to go into epithelial cells that make up blood vessels, monocytes that differentiate into t cells and b cells, and hitches a ride with neutrophils. When pathogens are inside cells, they’re hidden from the immune system.
Extracellular enzymes:
Coagulase (Staphylococci) - clot blood; isolate bacteria from host
Process that enable fibrinogen to form into fibrin which makes clots. The formation of fibrin causes isolation from the host and its immune system like a protective cocoon.
Kinases - destroy blood clots
e.g., streptokinase
Allows for pathogen to spread as it breaks clots and allows pathogen to go into previously clotted injuries which then allows it to gain access to the inside of our bodies.
Hyaluronidase - hydrolyzes hyaluronic acid, polysaccharide bridging cells of connective tissue → allows microbes to spread (streptococcus)
breaks apart the chemical fibers that connect your connective tissues which allows the pathogen to penetrate those tissues
Collagenase - digests collagen; in connective tissue of muscle, organs, tissues. Similar to Hyaluronidase
Protein A (Staphylococcus) - Acts as an Fc receptor
Neutralizes antibodies as they’re bound on the wrong end (the Fc receptor).
Proteases - destroys host proteins; IgA protease
Antigenic variation:
Alteration of pathogen surface proteins; possess alternate genes
The immune body can’t respond right away as the antigens are changed. Allows for the pathogen to go undetected.
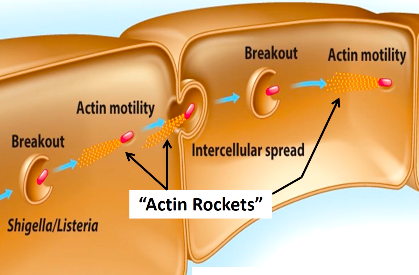
Cytoskeleton:
Some pathogens enter cells of the host using filaments of the cytoskeleton; actin filaments are common means of entry
Exploits the host cytoskeleton
Invasins (Salmonella) - pathogen surface proteins that rearrange actin filaments → induces membrane ruffling (create more membrane folds); pathogen will be engulfed into cell
While in a cell, particularly those that are non-motile, will take actin monomers and binds to the cell and polymerizes, propelling the bacterium through cell cytoplasm and into other cells
This can cause damage to the cells
If a pathogen breaches the host defenses, it can damage the host by:
Using the host’s nutrients
pathogen acquire host’s iron via siderophores
Iron (Fe) chelators - bind iron
Causing direct damage in the vicinity of infection
Intracellular pathogens, e.g., viral infection
Production of toxins: transported by blood, lymph
inhibit protein synthesis
disruption of membrane
Introduction of hypersensitivity reactions: overproduction of cytokines
Exotoxins - secreted to the surrounding environment
Toxins produced by the pathogen and secreted outside and defuses into blood, tissue, etc
Water soluble proteins (many are enzymes); most are plasmid-based or in phages (lysogenic conversion)
Action: destroy specific host cell structures or inhibit metabolic functions; can be very lethal
Types: A-B toxins, Membrane-disrupting toxins, Superantigens
Antitoxins: toxoid - forms provide immunity
A-B toxins - consists of an active enzyme component (A) and a cell binding component (B)
These toxins have specificity for certain cell types and will bind to those cells and cause damage to them
Types:
Diphtheria toxin (Corynebacterium diphtheriae): inhibits protein synthesis; phage carries tox gene
Botulism toxin (Clostridium botulinum): neurotoxin; prevents nerve impulses to muscles; causes flaccid paralysis
Tetanus toxin (Clostridium tetani): neurotoxin tetanospasmin; blocks inhibitory nerve impulses to muscles; causes spasmodic contractions
Vibrio enterotoxin (Vibrio cholerae): cholera toxin; causes cellular secretion of fluids & electrolytes → severe diarrhea & dehydration
Membrane-Disrupting Toxins - cause lysis of host cells by disrupting plasma membrane by forming protein channels in membrane or by disrupting phospholipids
Leukocidins: kill phagocytic white blood cells
Hemolysins: kill red blood cells
Superantigens - Stimulate intense immune response of T cells
Produces a hypersensitive effect on the immune system
T cells stimulated to produce cytokines; regulates immune response
Excessive cytokine levels enter blood streams and induce symptoms; can lead to shock & death
Erythrogenic toxins (Streptococcus pyogenes): damage blood capillaries under skin → rash; scarlet fever
Staphylococcal enterotoxin (S. aureus)
Endotoxins - lipid portion (lipid A) of the LPS (lipopolysaccharide) layer of gram-negative bacteria → released when cells die and lyse
Not secreted but simply a part of the lipid A portion of the LPS of a gram-negative bacteria
Exert effect by stimulating by stimulating macrophages to release toxic levels of cytokines
Can also activate blood clotting proteins and induce fever
Endotoxic shock → drastic drop in blood pressure
Pathogenic Properties of Viruses
Evading hosts → grow inside host cells
Cytopathic effect: visible effects of viral infection
Cytocidal effect: results in cell death
Cytocidal viruses stop host cell biosynthesis & induce cell’s lysosomes to release contents
Inclusion bodies/granules in some infected cells
Are usually viral parts (nucleic acids, proteins) to be assembled, can be diagnostic
EX: Rabies
Infected cells may fuse to form multinucleate syncytium (a giant cell)
When an enveloped virus enters a cell through membrane fusion, it may leave behind its viral envelope w/ proteins on the cell membrane. Other cells may fuse with the infected cells through the viral envelope left behind on the cell membrane, forming a giant cell.
Oncogenic viruses transforms cells into cancerous cells
Some virus-infected cells form interferons, protects non-infected cells
Viral infection reduces antigenic changes on the cell surface - rid body of infected cells