Yr9- C1: Atomic structure
C1.1
All substances are made of atoms.
An atom is the smallest part of an element that can
exist.
Compounds are formed from elements by chemical
reactions.
Compounds contain two or more elements chemically
combined in fixed proportions and can be represented
by formulae using the symbols of the atoms from
which they were formed.
Compounds can only be separated into elements by
chemical reactions.
Atoms:
An atom is the smallest part of an element that can exist.
All substances are made from atoms.
All atoms are made up of a tiny nucleus with electrons orbiting around it.
Elements:
Elements are made from one type of atom.
It is a pure substance.
Solids + Liquids and Gases
At temperatures above their boiling points, substances exist as gases
At temperatures above their melting points, but below their boiling points, substances exist as liquids
At temperatures below their melting points, substances exist as solids
Compounds:
A compound contains two or more elements chemically combined in fixed proportions.
pure substance
Mixtures:
A mixture is two or more elements or
compounds mixed together but not
chemically bonded.
Impure substances.
Molecules:
A molecule is two or more atoms chemically
bonded together.
C1.2
In chemical equations, the three states of matter are
shown as (s), (l) and (g), with (aq) for aqueous solutions.
The law of conservation of mass states that no atoms are
lost or made during a chemical reaction so the mass of the
products equals the mass of the reactants.
What is a chemical reaction?
A chemical reaction is a chemical change that takes place
when the atoms in the reactants get rearranged to form new
substances
called products
Law of conservation of mass:
In a chemical reaction, the mass of the reactants is always
the same as the mass of the products.
This is because atoms are not created or destroyed in
chemical reactions; they are just rearranged into different
compounds.
total mass of reactants = total mass of products
C1.3 Separating Mixtures
A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged.
mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography
Compounds:
fixed composition e.g water is always H2O
There are chemical bonds between atoms of different elements
A chemical reaction is used to separate the elements in a compound.
Mixtures:
No fixed composition (varying proportions)
No chemical bonds between atoms of different substances in a
mixture
Separated by physical means using differences in properties of each substance in the mixture.
Filtration:
Separates insoluble solids from those that are soluble in the solvent (liquid), -e.g sand from salt solution.
Crystallisation:
Separates soluble solids from the solvent by evaporating the solvent,
e.g.solid salt from salt solution.
Simple distillation:
Distillation is a process that can be used to separate a pure liquid from a mixture of liquids or a solid. It works when the liquids have different boiling points.
The sequence of events in distillation:
heating → evaporating → cooling → condensing
C1.4 Distillation and Paper Chromatography:
filtration: separates insoluble from soluble substances
distillation: separates substances with different boiling points
soluble: a substance that does dissolve
insoluble: a substance that does not dissolve
mixture: two substances are mixed but not chemically combined
compound: atoms of two or more elements that are chemically combined
crystallisation: used to obtain a soluble substance from a solution
Paper chromatography:
Paper chromatography can be used to:
distinguish between pure and impure substances
identify substances by comparing the pattern on the chromatogram with the patterns formed by known substances
identify substances by calculating their Rf values.
Stationary phase –chromatography paper
(doesn’t move)
Mobile phase- the solvent used
It works because some of the coloured substances dissolve in the solvent better (Have a greater AFFINITY for the solvent) than others, so the solvent carries this substance further up the paper.
Other substances have a greater AFFINITY for the stationary phase and do not move as much if at all.
Calculating Rf Values:
Rf= distance moved by compound/ distance moved by solvent
4.0/5.5= 0.73
Miscible liquids:
capable of mixing
e.g alcohol and water, or crude oil
fractional distillation: separate miscible liquids (and gases) based on difference in their boiling points
Fractional distillation uses an
extra fractionating column for
better separation of liquids
with similar b.p.
Fractional and Simple distillation:
Simple Distillation is a process that separate a pure liquid from a mixture of miscible liquids e.g. ethanol and water.
Fractional distillation takes advantage of the fact that different liquids have different boiling points.
heating → vapourising → cooling → condensing
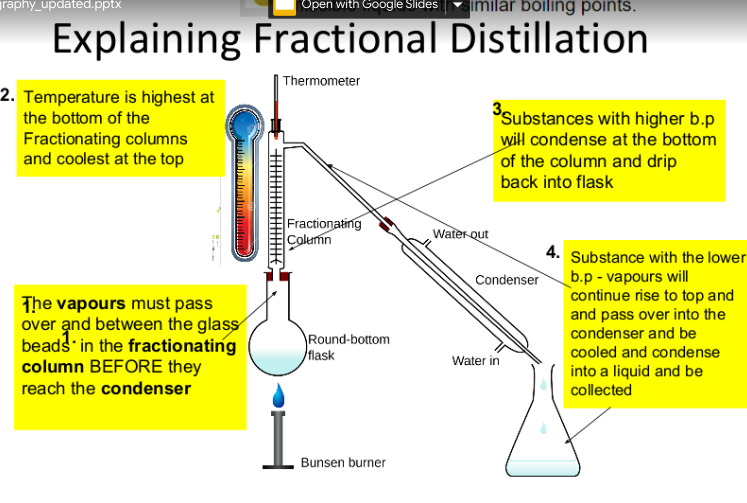
Miscible: Liquids that dissolve in each other and mix completely
Condenser: Apparatus that cools down gas and turns it into a liquid (condenses)
Fractional Distillation: Method used to separate many liquids with similar boiling points
Simple Distillation: Method used to separate two liquids with different boiling points
Fractionating Column: Apparatus for separating many columns with different boiling points
C1.5 History of the atom:
 Democritus:
Democritus:
All matter could be divided and sub-divided into smaller and smaller units, until you got to a particle that could not be divided any further - an atom.
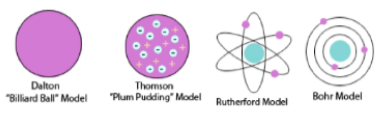
John Dalton:(1766-1844) – 1803
All matter made up of:
tiny, indivisible, solid spheres called atoms
Atoms could not be created or destroyed (Law of Conservation of mass)
Atoms could join together to form larger particles in compounds.
Billiard Ball model
 -
-John Jacob Berzelius: (1779- 1848)
all atoms have different weights, the
relative masses he calculated are very
close to the ones used today.
atoms joined together in fixed
proportions.
represented the atoms by chemical
symbols.
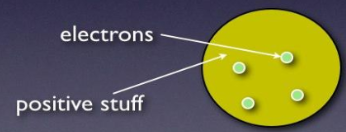
JJ Thomson: (1856-1940)
Discovered the electron
Proposed the plum pudding model (1899)
was a ball with positive charge with negative electrons embedded in it
Positive and negative charges balance to make atom neutral, measured the mass of an electron and found it to be 1/1840 of an atom (hydrogen)
Concluded mass of an atom was due to the nucleus not the electrons.
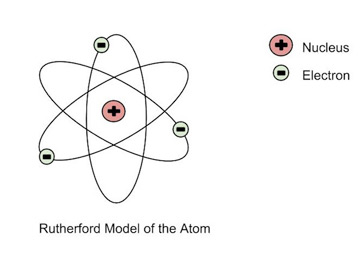
Ernest Rutherford: (1871- 1937):
Proposed the nuclear model.
an atom is a small nucleus with orbiting electrons with spaces between them
Rutherford bombarded atoms of a thin gold foil with positively charged alpha particles.
he found that most of the positive particles went straight through, showing the atom was mostly space.
some particles bounced straight back, as if they were hitting something solid
He concluded that there must be a tiny dense positively charged centre in the atom which repel the positive particles. He called this the nucleus.
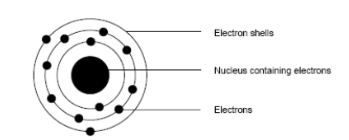
 Niels Bohr: (1885-1962)
Niels Bohr: (1885-1962)
concluded electrons were arranged in definite shells or energy levels around the nucleus.
Each shell could only hold a certain number of electrons,
when one was full, the next began to fill up. He called this
electronic configuration.
He stated that atoms with full shells were not very reactive, and that it was the electrons that determined the reactivity of the atom.
 James Chadwick: 1932
James Chadwick: 1932
He discovered the neutron, a particle with no electrical charge but have the same mass as a proton that is also found in the nucleus of a atom
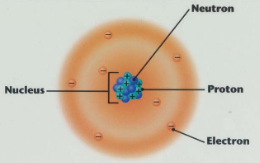
C1.6: Structure of the atom:
Atoms are the basic building block of all substances
There are smaller subatomic particles in an atom
Protons
Electrons
Neutrons
Inside the nucleus there are Protons and Neutrons
Protons have a charge of +1. Protons have a mass of 1
Neutrons have a charge of 0 (no charge). Neutrons have a mass of 1.
Around the nucleus are the Electrons in shells.
Electrons have a negative charge. -1
Electrons have very little mass. It is negligible. 1/2000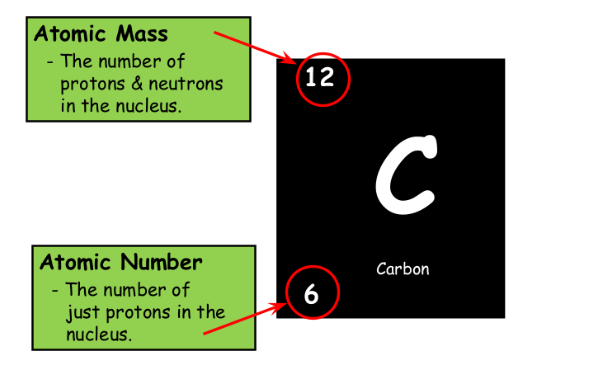
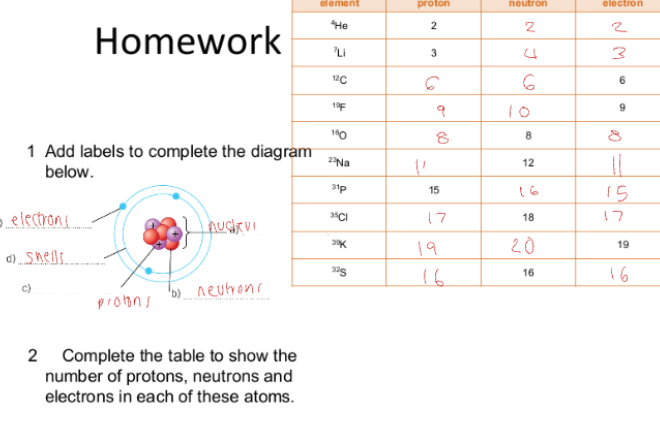
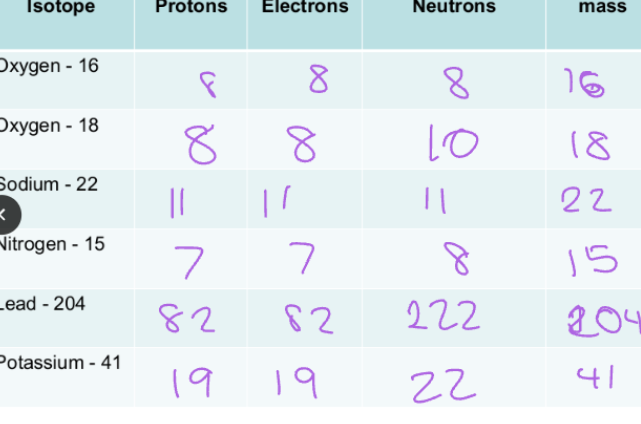
C1.7 Ions, atoms and Isotopes:
Ions:
Atoms can lose of gain electrons.
An atom that has lost of gained one of more electrons is called an ion.
A positively charged ions (lost electrons) is called a cation.
A negatively charged ion (gained electrons) is called an anion.
Electrons exist in shells around the nucleus.
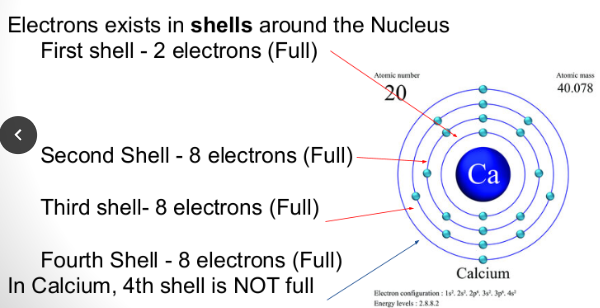 Atoms want to lose or gain electrons for the atom to be stable. This happens when that outer shell is a full shell of electrons.
Atoms want to lose or gain electrons for the atom to be stable. This happens when that outer shell is a full shell of electrons.
For example, Calcium will lose two electrons for the shell to be full.
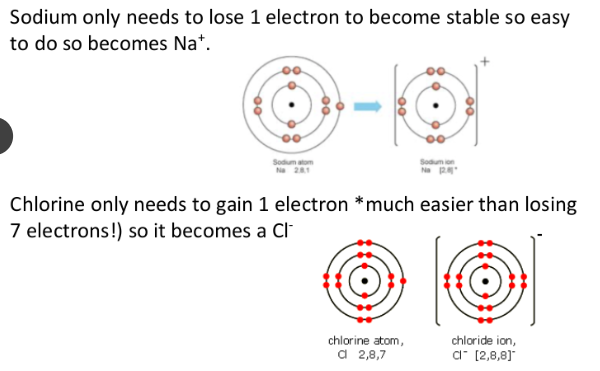 E.g Sodium. → Sodium can lose one electron in its outer shell
E.g Sodium. → Sodium can lose one electron in its outer shell
→This means it has lost one negative charge.
→ So the sodium ion becomes positively charged because it still has 11 protons (positive charges) in the nucleus.
E.g Chlorine → Chlorine can gain one electron in its outer shell.
→This means it has gained one negative charge
→ So the chlorine ion becomes negatively charged because it still has 17 protons in the nucleus.
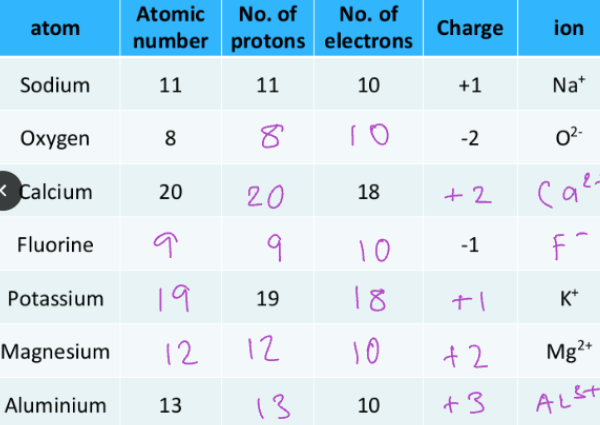
Isotopes:
Elements are made up of one type of atom.
Atoms of the same element always have the same number of protons, but they may have different number of neutrons. (Isotopes)
The charge will not change but the atomic mass will change.
Isotopes have unique properties, and these make them useful in diagnostics and treatment applications. e.g nuclear medicine
Properties of Isotopes:
Chemical properties of Isotopes are the same.
This is because they have the same number of electrons and protons in their outermost shell.
Physical properties of Isotopes are different.
Melting point and density are different.
Calculating Atomic Mass:
Percent% abundance of isotopes
Mass of each isotope of that element
Weighted average=
Example. 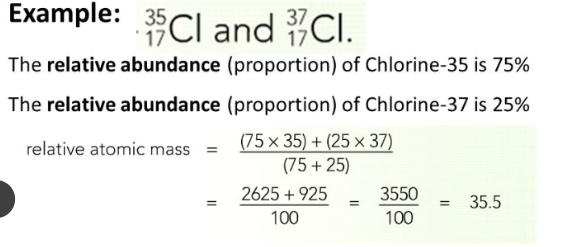
C1.8 Electronic Configuration
Each shell or energy level can be thought of as a region
where electrons will be found.
An energy level or shell can only exist if there is an
electron in it.
Electrons will start to fill the shell with the lowest
energy (nearest the nucleus) first.
A new shell only starts to fill when the previous one is
full.
Nucleus:
Dense – it contains nearly all the mass of the atom in a tiny space.
Made up of protons and neutrons.
Positively charged because of the protons.
Electrons:
Thinly spread around the outside of the atom.
Very small and light.
Negatively charged.
Found orbiting the nucleus in layers called shells.
Able to be lost or gained in chemical reactions.
Electrons are not evenly spread but exist in layers called shells. (The shells can also be called energy levels).
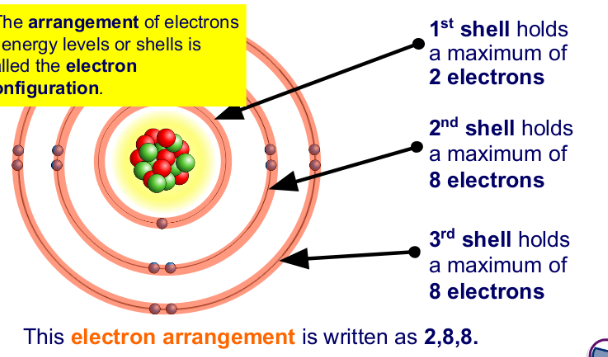 The group number = the no.of electrons in the outside shell;
The group number = the no.of electrons in the outside shell;
The period number tells us how many electron shells an atom has
 Knowt
Knowt
