7.2 Equilibrium Law and Equilibrium Constants
- Two types of equilibria:
- A homogenous equilibrium has everything present in the same phase.
- The usual examples include reactions where everything is a gas, or everything is present in the same solution.
- A heterogenous equilibrium has matter present in more than one phase.
- The usual examples include reactions involving solids/liquids and gases, solids and solutions.
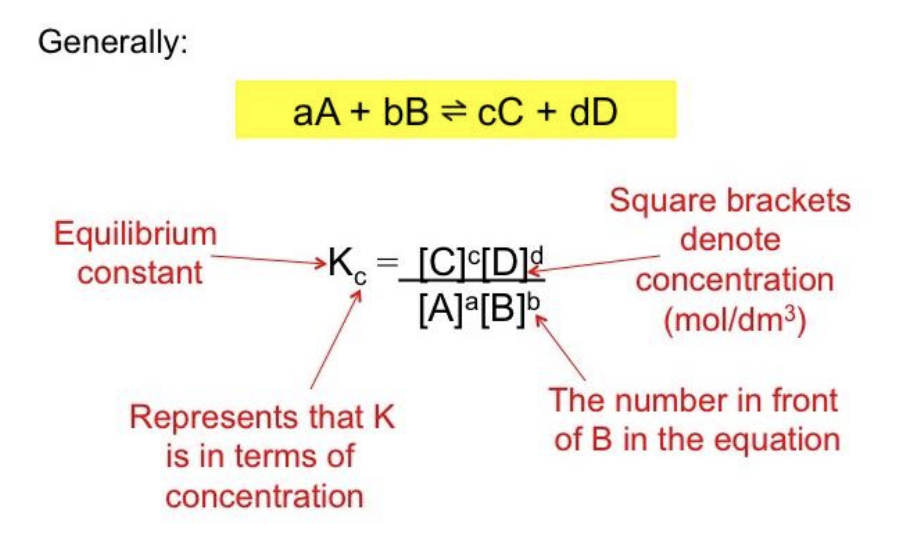
Equilibrium Law
- The mathematical description of a chemical system at equilibrium.
- aA + bB → cC + dD
Equilibrium Constant, K
A constant numerical value that defines the equilibrium law for a system.
The Haber Process Equilibrium (homogenous system)
To Calculate the K, plug in concentration values for each entity at equilibrium. Note that K does not have units.

Writing an expression for K in a heterogenous system
- Leave out solids and liquids.
K & K’
- K = 1/K’
- K’ = 1/K
- K = [products] / [reactants]
- If K >>> 1 – the reaction is favoured in the forward direction (at equilibrium, there’s a higher concentration of products than reactants
- If K = 1 – the equilibrium overall concentrations of products and reactants are equal
- If K <<< 1 – the reaction is favoured in the reverse direction (at equilibrium, there’s a higher concentration of reactants than products.