Chapter 19: Cell Junctions and the Extracellular Matrix
Cell-Cell junctions
- Cell-cell junctions are specialized structures that mediate interactions and connections between adjacent cells.
- They play a crucial role in maintaining tissue integrity, providing mechanical strength, and facilitating communication between cells.
Tight Junctions:
- Tight junctions (also known as occluding junctions) form a seal between adjacent cells, preventing the passage of molecules and ions between the cells.
- They are composed of transmembrane proteins, including claudins and occludins, which interact with proteins in neighboring cells.
- Tight junctions are primarily found in epithelial and endothelial tissues and contribute to the establishment of tissue barriers.
Adherens Junctions:
- Adherens junctions are involved in cell-cell adhesion and provide mechanical strength to tissues.
- They are characterized by the presence of cadherin proteins, which mediate calcium-dependent homophilic interactions between adjacent cells.
- The intracellular region of cadherins interacts with cytoplasmic proteins, such as β-catenin and α-catenin, which link the junctions to the actin cytoskeleton.
- Adherens junctions are critical for maintaining tissue integrity, cell shape, and cell migration during development and wound healing.
Desmosomes:
- Desmosomes are strong intercellular junctions that provide mechanical stability and resistance to shearing forces.
- They are composed of desmogleins and desmocollins, which are transmembrane proteins that interact with cadherin-like proteins in adjacent cells.
- Inside the cell, desmosomal cadherins are linked to intermediate filaments, such as keratin filaments, which extend throughout the cytoplasm.
- Desmosomes are particularly abundant in tissues subjected to mechanical stress, such as the skin, heart, and uterus.
Gap Junctions:
- Gap junctions are specialized channels that allow direct communication and exchange of small molecules between adjacent cells.
- They are composed of connexin proteins, which form hemichannels called connexons in the plasma membrane.
- Connexons from two neighboring cells align and dock together, creating a pore that facilitates the passage of ions, small metabolites, and signaling molecules.
- Gap junctions are involved in coordinating cellular activities, electrical signaling, and metabolic coupling in tissues like cardiac and neural tissues.
Hemidesmosomes:
Hemidesmosomes anchor epithelial cells to the underlying extracellular matrix, providing stability and mechanical support.
They are composed of transmembrane proteins, such as integrins, which interact with extracellular matrix components like laminin and collagen.
Inside the cell, hemidesmosomal proteins are linked to intermediate filaments, specifically keratin filaments.
Hemidesmosomes are particularly important in tissues subjected to mechanical stress, such as the skin and epithelial linings of organs.

The Extracellular Matrix of Animals
- The extracellular matrix (ECM) is a complex and dynamic network of macromolecules found in the extracellular space of animal tissues.
- The ECM provides structural support, mechanical strength, and biochemical cues to cells within tissues.
- Major components of the ECM include fibrous proteins, glycosaminoglycans (GAGs), proteoglycans, and glycoproteins.
- Collagen is the most abundant fibrous protein in the ECM, forming strong and flexible fibrils that contribute to tissue integrity and strength.
- Other fibrous proteins in the ECM include elastin, which provides elasticity to tissues, and fibronectin, which plays a role in cell adhesion and signaling.
- GAGs are long, unbranched polysaccharide chains that interact with proteins to form proteoglycans. They provide hydration, support, and resilience to the ECM.
- Proteoglycans consist of a core protein to which GAG chains are attached. They help maintain the ECM structure, regulate cell behavior, and bind growth factors.
- Glycoproteins, such as laminin and fibronectin, are involved in cell adhesion, migration, and signaling within the ECM.
- The ECM is organized into specialized structures, such as basement membranes and fibrillar networks, which vary depending on the tissue type and function.
- Basement membranes are thin, sheet-like structures that underlie epithelial and endothelial tissues, providing a scaffold for cell attachment and separating tissue compartments.
- Fibrillar networks, composed of collagen and other fibrous proteins, provide structural support and organization in connective tissues like tendons, cartilage, and bone.
- Cells interact with the ECM through cell surface receptors, including integrins, which mediate adhesion and transmit signals bidirectionally between the ECM and the cell.
- Integrins and other ECM receptors can activate signaling pathways that regulate cell proliferation, migration, differentiation, and survival.
- Remodeling of the ECM is tightly regulated by various enzymes, including matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), to maintain tissue homeostasis and repair.
Cell-Matrix Junctions
- Cell-matrix junctions are specialized structures that mediate the interaction between cells and the extracellular matrix (ECM).
- These junctions play crucial roles in cell adhesion, signaling, migration, and tissue development.
- The main types of cell-matrix junctions include focal adhesions, hemidesmosomes, and podosomes.
Focal Adhesions:
- Focal adhesions are large, dynamic structures that connect the ECM to the actin cytoskeleton within cells.
- They are formed by the clustering of integrin transmembrane receptors at specific sites on the plasma membrane.
- Integrins, such as α and β subunits, bind to ECM components, such as fibronectin or collagen, through their extracellular domains.
- The intracellular domains of integrins interact with a variety of adaptor proteins, including talin, vinculin, and focal adhesion kinase (FAK).
- Adaptor proteins link integrins to the actin cytoskeleton through interactions with actin-binding proteins like actinin, paxillin, and α-actinin.
- Focal adhesions transmit mechanical forces, regulate cell adhesion strength, and activate intracellular signaling pathways, including those involved in cell proliferation, migration, and survival.
- Signaling molecules, such as kinases, GTPases, and focal adhesion-associated proteins, are recruited to focal adhesions, modulating cellular processes and responses to the ECM.
Hemidesmosomes:
- Hemidesmosomes are specialized junctions that anchor epithelial cells to the underlying basement membrane.
- They are primarily found in tissues subjected to mechanical stress, such as the skin and mucosal linings.
- Hemidesmosomes consist of transmembrane integrin α6β4 receptors, which interact with laminins and collagen within the basement membrane.
- Intracellularly, integrin α6β4 associates with plectin and BPAG1, which connect to intermediate filaments, such as keratins.
- The anchorage provided by hemidesmosomes helps maintain tissue integrity, resist mechanical forces, and regulate epithelial cell polarity.
Podosomes:
- Podosomes are dynamic, actin-rich structures found in specialized cells like osteoclasts and macrophages.
- They function as sites of cell adhesion, extracellular matrix degradation, and cell migration.
- Podosomes are characterized by a core of actin filaments surrounded by a ring of adhesion molecules, such as integrins and vinculin.
- Proteolytic enzymes, like matrix metalloproteinases, localize to podosomes and facilitate ECM remodeling.
- Podosomes play roles in tissue remodeling, immune response, and cell invasion processes.
Regulation and Signaling:
- Cell-matrix junctions are regulated by numerous signaling pathways, including those involving integrin activation, cytoskeletal rearrangement, and kinase signaling.
- Various intracellular signaling molecules, such as focal adhesion kinase (FAK), Src family kinases, and small GTPases (e.g., Rho, Rac, and Cdc42), are involved in the regulation of cell-matrix junctions.
- Signaling through cell-matrix junctions can influence cell behavior, including adhesion strength, migration speed, proliferation, differentiation, and survival.
- Dysregulation of cell-matrix junctions and associated signaling pathways can contribute to disease conditions, including cancer metastasis, tissue fibrosis, and autoimmune disorders.
The Plant Cell Wall
The plant cell wall is a complex and rigid structure that surrounds the plasma membrane of plant cells.
It provides structural support, mechanical strength, and protection to plant cells and tissues.
The plant cell wall is primarily composed of cellulose, hemicelluloses, pectins, and lignin.
- Cellulose, a polysaccharide made up of glucose units, forms long, linear chains that are bundled together to create strong microfibrils.
- Hemicelluloses are a diverse group of polysaccharides that interact with cellulose, providing cross-linkages and contributing to the flexibility and stability of the cell wall.
- Pectins are complex polysaccharides that form a gel-like matrix between cellulose microfibrils, imparting adhesive properties and regulating cell-cell communication.
- Lignin is a complex phenolic polymer that fills the spaces between cellulose microfibrils, providing rigidity and hydrophobicity to the cell wall.
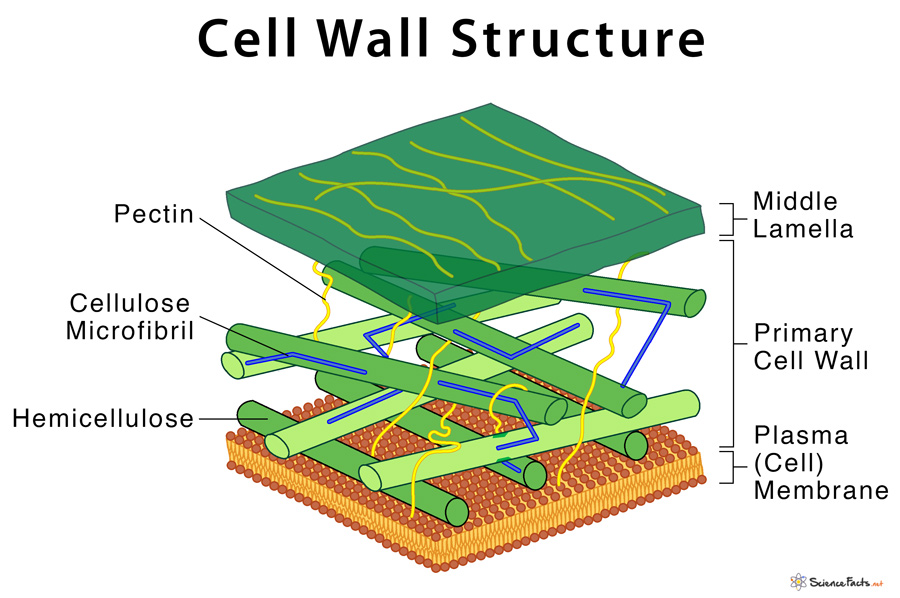
In addition to these major components, the plant cell wall may also contain proteins, enzymes, lipids, and other secondary metabolites.
The cell wall is organized into primary and secondary cell walls, each with distinct properties and functions.
The primary cell wall is flexible and extensible, allowing cell growth and expansion during development.
As cells mature, some plants develop a secondary cell wall inside the primary cell wall, which provides additional strength and protection.
The secondary cell wall is often thicker and contains a higher proportion of lignin, making it more rigid and impermeable.
The plant cell wall interacts with the cytoskeleton and plasma membrane, providing structural support and facilitating cell-cell adhesion.
The cell wall also acts as a barrier against pathogens, pests, and environmental stresses.
Plant cell walls have specialized regions called plasmodesmata, which are channels that connect adjacent cells, allowing the exchange of molecules and signaling between cells.
The composition and structure of the cell wall can vary across different plant tissues and cell types, allowing for functional diversity.
The synthesis, modification, and degradation of the cell wall are tightly regulated by various enzymes, transporters, and signaling pathways.
Plant cell walls are dynamic structures that can remodel in response to developmental and environmental cues.