1.4F Dilutions
Diluting a Solution
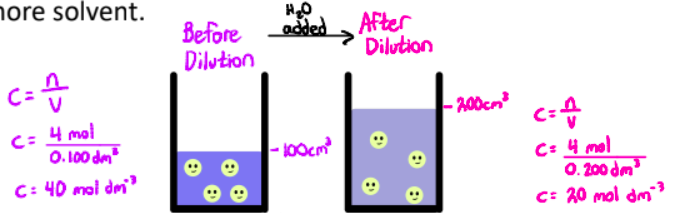
Dilution is the process of decreasing the concentration of a solution by adding more solvent.
The amount of solute in the solution is the same before and after the dilution.
Dilution Equation
C1V1 = C2V2
C1 = initial concentration
V1 = initial volume
C2 = final concentration
V2 = final volume