Diffusion
Transport processes
These processes are important in functioning of body. In gas exchange between lungs and bloodstream, oxygen and nutrient transport to tissues and cells from bloodstream, waste products of metabolism is transported to the blood. Therapeutic approach for absorption of drugs and dialysis.
Phenomenon of diffusion
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration over time by random thermal motion.
• On the macroscopic level:
Add a drop of food colouring to a glass of hot water and to another glass of cold water, after few seconds we observe that the drops in the hot water happens faster. We derive a homogeneous distribution.
• On a microscopic level:
A container split into two parts, the molecules in both sides are in constant motion and collide with themselves and the wall of the container. When we increase the temperature the molecules move faster.
Causes
Due to the uneven(inhomogeneous) distribution of particles.
Consequences
The particles move from a solution of higher concentration to lower concentration till we achieve a homogeneous distribution.
Quantitative description
A cross-section between the high concentration and low concentration due to concentration difference (concentration gradient).
Conc. gradient = conc. difference/distance = <0
Matter flow velocity(I) is number of particles that pass within a given time.
I = change in moles/change in time
Matter flow density (J) is amount of particles with time divided by area(A).
J = change in moles/change in time x A
Fick’s 1st law matter flow density is linearly proportional to concentration and diffusion gradient.
- increase in concentration gradient results increased diffusion rate.
- particle properties determining diffusion coefficient.
Stokes-Einstein equation,Diffusion coefficient
In spherical particles, environmental factors relate with the diffusion coefficient. Increase in temperature leads to increased diffusion coefficient and decreased particle size and viscosity.
D = 𝑘/6𝜋 × 𝑇/𝜂𝑟
• temperature (T);
the higher the temperature, the faster the thermal motion
• geometry/shape of the particle (r);
small particle diffuse more easily than big particle.
• viscosity of the medium (η);
diffusion is faster in low viscosity media than in high viscosity media.
Boltzmann constant k = 1.38 × 10−23 J/K
𝐷(𝑂2) = 2 × 10^-9, number of particles diffusing through 1m^2 in 1s at unit of concentration gradient.
Relationship between diffusion time and mean displacement
Average distance(R) is proportional to the square root of Diffusion coefficient(D) × time.
R~√2𝐷𝑡 ×𝑡
It indicates that diffusion is faster for smaller particles.
Example: gaseous exchange between the blood and the lungs; for CO2 R is 1micrometer, D is 1.2 × 10^-8 time is about 0.5s.
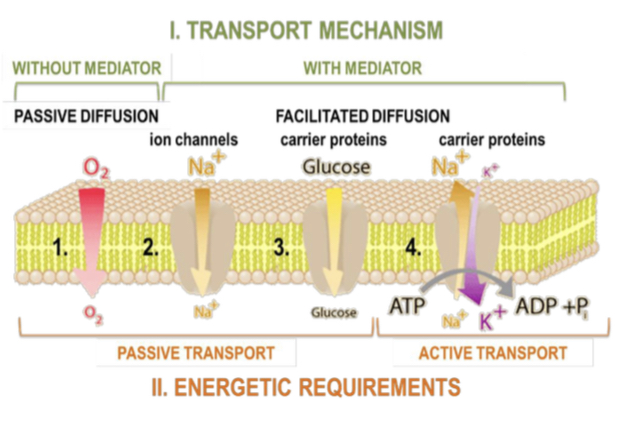
Transport processes across biological membranes
 Knowt
Knowt