Chapter 1- Measurement
INTRODUCTION TO PHYSICS:
- To compare and measure things in sciences, we need different units which are established by experiments.
- One purpose of physics is to design and conduct those experiments.
- These experiments bring us advancements and new technology such as the GPS which is the result of the dedication of physicists.
MEASURING THINGS
- Each physical unit is measured in its own unit by comparison with a standard.
- Units are unique names assigned to quantities.
- Standard corresponds to exactly 1.0 unit of that quantity.
- To ensure that there is no problem in organising quantities and we can work with them efficiently, we assign standards to base quantities only.
- Base quantities are those quantities on the basis of which other quantities can be expressed, e.g. speed is the ratio of length to time.
- All other physical quantities are then defined in terms of their base quantities, e.g. speed is m/s in terms of base quantities, length and time.
- It is ensured that the base standards are kept very precise.
THE SI UNITS
- In 1971, the standards for base quantities was formed known as the International system of units, or the metric system.
- To express very large quantities, scientific notation is used which is written to powers of 10, e.g. 3.5 x 10^5.
- On computers, it takes a briefer look with the notation expressed in the form of E where E stands for the exponent of 10.
CHANGING UNITS
- Much of the times, we convert units to different ones using a conversion factor.
- The quantity to be converted is multiplied by a conversion factor, e.g. hours are multiplied by 60 to convert to minutes, and meters is multiplied by 1/1000 to convert it to kilometers.
- In conversion, the units are always canceled.
DIFFERENT QUANTITIES
LENGTH:
- In 1792, earth standard (between northpole and the equator) was defined to be the meter.
- However, for practical reasons, the meter became known as the distance between the lines on the ends of a platinum–iridium bar, called the standard meter bar.
- In 1960, a new standard for the meter, based on the wavelength of light, was adopted, and the previous standard was abandoned.
- As the world progressed however, even the standard of krypton measurement was not termed as precise, and the meter was redefined as the distance traveled by light in a specified time interval, which is very precise since the speed of light, c, is always precisely 292 798 458 m/s.
TIME
- In scientific language, time is the measure of how long an event lasts.
- Any phenomenon that repeats itself is a possible time standard.
- Earth’s rotations have been used for estimating time in many devices such as Quartz clocks but it was not very precise.
- For more precision, atomic clocks have been adopted which work based on the jumping of electrons within atoms.
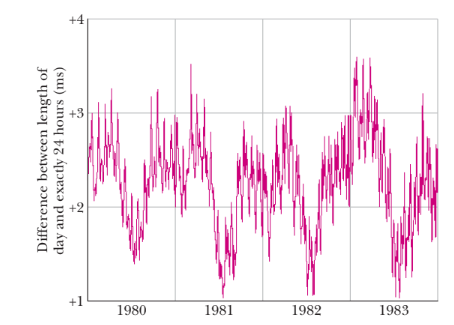
- The graph of the rotations of the earth show that it is varying and therefore, doesn’t provide a very accurate time measurement.
MASS
- The SI standard of mass is a cylinder of platinum and iridium which has been agreed upon to be a mass of 1 kilogram.
- Accurate copies have been sent all around the world.
- However, the mass of atoms can only be precisely compared to eachother.
- Therefore, the carbon-12 atom is used as a unit for measuring mass of atoms and basically means 12 atomic units.
- Density is mass per unit volume and is typically measured as either grams per cubic centimeter or kilograms per cubic meter.
- The density of water which is 1000 g/cm^3, is used as the main comparison in densities and the density of anything compared with that of water is called the relative density.
PREFIXES
- In physics, to represent large quantities, bases of 10 are used.
- The following prefixes are used to represent the different powers of 10.
| PREFIX | POWER OF 10 |
|---|
| Pico | 10^-12 |
| Nano | 10^-9 |
| Micro | 10^-6 |
| Milli | 10^-3 |
| Kilo | 10^3 |
| Mega | 10^6 |
| Giga | 10^9 |
| Tera | 10^12 |
