chemotherapeutic agents drugs
grSummary of drugs
Anti-bacterial
Drug | Type of agent | What does it work on | Example name | |
|---|---|---|---|---|
T | Tetracycline | Antibacterial | 30S subunit | Doxycycline |
C | Chloramphenicol | antibacterial | 50S subunit | |
A | Acridines | Antibacterial | alternates the base-pairing properties | |
AD | Actinomycin D | Antibacterial | inhibits RNA polymerase | Dactinomycin |
M | Metronidazole | Antibacterial | Works directly on the DNA | Flagyl |
F | Fluoroquinolones | Antibacterial | Inhibits DNA gyrase | Ciprofloxacin |
S | Sulphonamides | Antibacterial | Inhibits folate synthesis | Sulfadiazine |
Tyrocidines & Gramicidin A | Antibacterial | Disorganize membrane structure | ||
Polymyxins | Antibacterial | Disorganize membrane structure | Polymyxin B | |
Beta-lactam | Antibacterial | Inhibits cell wall synthesis | Penicillin (naficillin) | |
Isoniazid | Anti Mycobacterial | Inhibits mycoacid synthesis | Isoniazid | |
Rifampicin | Anti Mycobacterial | Inhibits RNA synthesis | Rifampin |
Cancer
Drug | Type of agent | What does it work on | Cell cycle dependent? | Type of cancer |
|---|---|---|---|---|
Cisplatin | Alkylating agent | Alters the double helix shape of DNA | No | Testicular cancer |
Fluorouracil | Antimetabolite | Inhibits pyrimidine thymidine synthesis | yes | leukaemia, breast, ovary, GIT etc |
Vincristine | Plant derivatives | Inhibits microtubule | yes | Leukaemia, neuroblastoma, lymphomas |
Actinomycin C, Mitomycin | Antineoplastic antibiotics | Interrelate between DNA bases - breaks DNA bonds | no | Leuk, lymph, breast, GIT, ovarian, bladder, lung etc |
Doxorubicin | Anthracycline (antineoplastic) | Inhibits topoisomerase II -> interchelate into DNA -> block DNA & RNA synthesis -> generates free radicals | no | Wide use |
Cortisol | Hormonal | Regulates carbohydrate metabolism & anti inflammatory effects | no | Therapy & pallitative care |
Oestrogen | Hormonal | Negative feedback inhibition of GnRH secretion from HT | Pallitative treatment of adrogen-dependent prostatic tumourse | |
Progestogens | Hormonal | Inhibits endometrial cell growth & causes cell differentiation | Advanced endometrial cancer | |
Tamoxifen | Selective Oestrogen Receptor Modulators & Antioestrogens | Blocks actions of oestrogen in breast tissue -> inhibits growth of Bca | Breast cancer (Bca) | |
Fulvestrant | Antioestrogens | Antagonist of oestrogen in all tissues | Progressive Bca | |
Anastrozole | Aromatase inhibitor | Inhibits conversion of androgens to oestrogen in the adrenal cortex | Advanced prostate carcinoma | |
Enzalutamide | Anti-androgen | Inhibits androgen binding to androgen receptors | prostate | |
Bevacizumab | Monoclonal ab | Neutralises VEGF: Prevents angiogenesis crucial for tumour survival | Colorectal cancer | |
Trastuzumab | Monoclonal antibodies | Binds to HER2/ERBB2 - will cause immune system to react | Breast cancer overexpressing HER2 |
Viruses
Drug | Virus | Type of agent | What does it work on |
|---|---|---|---|
Acyclovir | HSV-1 &-2, CMV, Varicella | Nucleoside analouge | Inhibits viral DNA polymerase by competing with endogenous nucleosides |
Ribabirin /taribavirin | Viral respiratory infections | Aguanoisine analouge | Inhibits viral DNA polymerase by competing with endogenous nucleosides |
Amantadine, rimantadine | Viral repsiratory (influenza A) | Inhibition of viral uncoating | Blocks viral H+-ion channel which prevents acidification of the virus-containing vesicles - the viral genome cannot be released into host cell |
Oseltamivir (Tamiflu) | Viral resp. (Inf. A & B), H1N1 | Inhibitor of viral release | Inhibits neuraminidase - prevents the release of budded virus into the cells |
IFN-alpha, beta and gamma | Hepatitis B&C, herpes, hairy cell leukaemia | Interferons | Supresses host cell proliferation, inhibits viral penetration, uncoating and replication, inhibits viral RNA translation (many S/E!!!!) |
Fungus
Drug | Names | Type of agent | What does it work on | Clinical uses |
|---|---|---|---|---|
Ergosterol polyenes | Amphotericin B | Anti-ergosterol | Binds ergosterol (forms pores in the membranes | Systemic infections (aspergillus, candida, cryptococcus) |
Ergosterol azoles | Fluconazhole, econazoel | Anti-ergosterol | Inhibits ergosterol synthesis, by inhibiting fungal oxidative enzymes | Broad |
Ergosterol Allylamines | Tarbinafine | Anti-ergosterol | Inhibits ergosterol synthesis & causes build-up of fungicidal intermediary (squalene) | |
Echinocandins | Echinocandin B | Cell wall | Inhibits synthesis of vital parts of fungal cell wall: B-1,3-D-Glucan | Candida, aspergillosis |
Griseofulcin | Nuclear division | Binds to polymerised microtubules, disrupts the mitotic spindle & blocks replication in mitosis | Prolonged treatment for skin & nail infections | |
Flucytosine | DNA replication | Inhibits thymidylate synthase and DNA synthesis | Yeast & cryptococcal meningitis |
Antimalarial drugs
Drug | Names | Type of agent | What does it work on |
|---|---|---|---|
4-aminoquinolines | Chloroquinine | Treat acute attack | Unclear… Inhibits haem polymerase: there’s no formation of haemozoin |
Quinoline-methanols | quinine | Treat acute attack | Same as chloroquinine |
Folate anti-metabolites | Dapsone/ Sulphones | Treat acute attack | Compete with PABA for dihydropteroate synthase |
Primaquine | Target parasites in liver | Unclear: Something that causes H2O2 which will kill the parasites at the site | |
Cloroquine, meflouqine, pyrimethamine, dapsone & doxycycline | (combination needed) | Block link between exo-erythocytic & erythocytic stages | |
Primaquine, proguanil | Preventing transmission | Destroys gametocytes → prevents transmission |
Antibacterial agents
Protein synthesis 30S
Tetracyclines
General facts
Discovered in 1940s, are bacteriostatic
Derived from streptomyces
2nd generation: doxycycline and minocycline
Mechanism of Action
- Goes through the outer membrane via passive diffusion and active transport in G+
- Transverses through OmpF & OmpC porin channels in G -
- Then active transport through cell membrane
- Binds to the 30S subunit: Competes with the Aminoacyl-tRNA on the A site, and thereby prevents formation of the polypeptide chain
Pharmacokinetics:
- Administration: Orally or IV (only doxycycline in clinical setting)
- Absorption - administered with dairy products - can form nonabsorbable chelates with mg, Fe & Ai cations.
- Distribution - Everywhere. Undergoes calcification in teeth, bones and tumours with high Ca2+ content. Only doxycycline & minocycline cross BBB
- Metabolism - first generation not metabolized, 2nd generation partly metabolised in liver
- Excretion - by kidneys in urine, accumulates in renal failure. 2nd gen: excreted in bile.
Spectrum of activity
- Very wide, including G+ & G- bacteria, mycoplasma, rickettsiae, spirochaetes, protozoa
- Were taken preventatively and therefore there has become a lot of resistance
Clinical use
- Peptic ulcer disease
- Lymes disease (e.g. borellia)
- Mycoplasma pnemoniae
- Cholera
- Chlamydia
- etc…
Side effects
- GIT disturbances: nausea, bowel upset
- Deposition in calcified tissues: lead to discoloration and hyperplasia of teeth
- Photosensitivty: can cause sunburn
- Hepatotoxicity: rare, pregnenant women
- Vestibular disfunction: dizziness, vertigo & tinnitus
- Haematolofic toxicity
- Fanconi syndrom: electrolyte imbalance
- Pseudotumor cerebri: hypertension in brain - headaches & blurred vision
Resistance
- Big problem!
- Efflux pumps
- Enzymatic inactivation, a bit rarer
- Ribosomol protection: blocking the tetracyclines from binding, distorting structure or dislodging tetracycline.
Protein synthesis 50S
Chloramphenicol
General facts
- First broad-specturm antibiotic discovered
- Chloromycetin in the US
- Bacteriostatic, but can be bactericidal at high concentrations
Mechanism of Action
- penetrates through facilitated diffusion
- Binds to the 50S subunit, causing a conformational change. This will slow the binding of the tRNA to the A-site, and inhibits transpeptidation process (movement of peptide chain).
- Obs - it competes in binding the ribosome with macrolides & lincosamide, so combination treatments have no benefits.
Pharmacokinetics:
- Administration - oral, IV or topical as ear & eye drops
- Activation - the oral & IV are prodrugs: they are activated by hydrolysis in small intestine (oral) or converted to active form in circulation (IV).
- Absorption - oral rapidly absorbed from GIT, peak blood conc. after 2hrs. IV, serum levels are dependent on the patients metabolism. Plasma T1/2 ~4hrs.
- Distribution - everywhere, 60% in blood because binds to plasma proteins, can accumulate in braintissue.
- Metabolism - by hepatic glucuronyl transferase into inactive metabolites in liver
- Excretion - renal tube and excrete in bile, ~10% of drug excreted unchanged
Spectrum of activity
- Salmonella
- Chlamydiae
- Rickettsiae
- Spirochetes
- Mycoplasma
Clinical use
- Last resort drug for serious & life-threatening infections because there’s high toxicity
- Systemic uses include typhoid fever, cholera, anaerobic infections etc.
Side effects
- Anemias, causes bone marrow depression
- Drug interactions, can inhibit some liver drugs and therefore prevent the metabolism of drugs
- Occular irritation and toxicity, can cause blurred vision
- Grey baby syndrome
- Gastrointestinal disturbances, e.g. nasuea, vomiting, diarreah
- CNS effects, e.g. headach, depression, confusion
Resistance
- Enzymatic inactivation through acetylation by chloramphenicol acetyltransferase
- Decreased permeability
- Presences / increased presence in efflux pumps: the bacteria pumps out the drug
- Ribosomal protection, due to modification of binding site
Nucleic synthesis
There’s five ways to interfere with nucleic acid synthesis:
- Alteration of the base-pairing properties of the template
- Inhibition of either DNA or RNA polymerase
- Direct effects on DNA itself
- Inhibition of DNA gyrase
- Inhibition of Nucleotide synthesis
Acridines: Alternation of base-pairing properties
General facts
- are intercalating agents: meaning that they produce mutations by getting in between adjacent bases in the DNA, and therefore distorting the 3D structure of the helix.
- Examples are proflavine and acriflavine
Mechanism of Action
- Intercalcates into the DNA
- This doubles the distance between the pairs, causing disruption in DNA synthesis
- Causes frameshift mutations and therefore prevents bacterial reproduction
Clinical use
- Used as antibacterial agent during WW2 against G+ bacteria
- Only as a surface disinfectant or treating superficial wounds nowadays.
Toxicity
- Super toxic - cannot be used systemically.
- It’s carcinogenic in animals, because it gets into your DNA and stays there.
Actinomycin D: Inhibition of DNA or RNA polymerase
General facts
- E.g. actinomycin D, pilcamycin
- Intercalcalating
Mechanism of Action
- Intercalates in the minor groove of double helix between guanine-cytosine. GC-rich regions are proliferation genes
- It interferes with movement of RNA polymerase along the gene, therefore preventing transcription and triggers apoptosis of the cell
Spectrum of activity
- High inhibitory effect on gram +, - and some fungi
Clinical use
- Not first line of treatment due to strong side effects
- Used in combination with surgery for treatment of Wilm’s tumour and other rare diseases
- Part of combination chemotherapy, because it kills all cells in your body :-)
Pharmacokinetics
- Administration: By I.V.
- Absorption: Poorly via GT, therefore IV
- Distribution - Fast into tissue, mostly in bone marrow & nucleated cells. No BBB, but crosses placenta. Is free floating in blood.
- Metabolism - minimally metabolized in liver
- Excretion - excreted via bile (50-90%) and urine
Side effects
- Irritating to tissues
- Gastrointestinal distress - abdominal pain, diarrhoea, nasua etc
- Hepatotoxicity - can cause liver injury
- Haematological toxicity - can cause bone marrow depression
- Carinogenicity - can cause cancer
- hypersensitivity
Metronidazole: Direct effects on DNA itself
General facts
Is an alkylating agent: contains a chemical group that produces highly reactive carbonium ion intermediates. These carbonium ions react with nucleophilic substances in the cell - especially with electron donors. It forms covalent bonds with bases in the DNA. It therefore prevents replication.
- Sold under name: Flagyl
- Bactericidal
Mechanism of Action
- Diffuses across the cell membrane via passive diffusion
- Is activated through reduction by intracellular transport proteins. This only happens in anaerobic cells - it is therefore relatively safe for humans.
- The nitro group of the molecule binds to the DNA. This causes loss of helical DNA and strand breakage -- preventing synthesis.
Spectrum of activity
- Used for anaerobic infections
- Can be used for antiprotozoal
- There’s not much effect on human cells or aerobic cells
Clinical use
- Used against anaerobic cocci and bacilli infections, e.g. of wound abscess and combination therapy against helicobacter pylori.
- Used against anaerobic infections after bowel surgery
- Etc
Pharmacokinetics:
- Administration - Oral & IV
- Absorption - Rapidly absorbed after both
- Distribution - Oral bioaailability almost 100%! Goes everywhere, crosses BBB, <20% bound to plasma proteins
- Metabolism - Hepatic metabolism (30-60%)
- Excretion - Kidneys in urine, some fecal elimination
Side effects
- GIT distress; cramps & nausea
- Neurotoxicity: dizziness, vertigo, seizures, numbness
- Dermatological effects: Rashes & hives
- Steven-Johnson syndrome: rare flu-like syndromes with rashes, only found in combination with mebendazole
Drug interactions
- Acohol: causing nausea, vomiting, cramps
- Anticoagulants - prolonged prothrombin time
- Cimetidine - prologons the half life metronidazole
Resistance
- Rare, can be caused by specific resistance genes.
Fluoroquinolones: Inhibition of DNA Gyrase
General facts
DNA gyrase is an essential bacterial enzyme that unwinds the helix. It’s a type of topoisomerase.
- Most used: Ciprofloxacin
- Bactericidal
Mechanism of Action
- Inhibits topoisomerases/DNA gyrases
- This will cause permanent gaps in the DNA strands. This will cause repair by exonucleases. This will lead to breakdown of DNA and irreversible damage → death of bacteria.
- In G negative: Topoisomerase II, that normally prevents supercoiling of the DNA. Here: There’s no effect on transcription or replication
- In G positive: Topoisomerase IV, that normally relaxes supercoiled DNA
Pharmacokinetics:
- Administration - Orally, IV and Topically
- Absorption - Well absorbed from GIT (80-90%). With IV, dietary supplements containing Fe, Zn or Ca interfere with absorption.
- Distribution - Widely distributed in all tissues. Plasma binding 10-40%, Penetration of BBB is low (except ofloxacin which crosses BBB well). Can accumulate in macrophages.
- Metabolism - Hepatic metabolism
- Excretion - primarily renal - renal failure can cause toxicity. Some bile excretion.
Spectrum of activity
- Against G- organisms
- E.g. mycobacteria & legionella pneumophila
- Less active against G+ due to resistance
Clinical use:
There is a lot of different types of fluoroquinolones that have different clinical uses:
- Nalidixic acid, norfloxacin: urinary tract infections
- Ciprofloxacin: main one used, mostly against G- bacteria, e.g. chlamydia.
- Levofloxacin: used against streptococcus pneumoniae e.g.
Side effects
- GIT: Diarrhoea
- CNS effects: Headache, dizziness, confusion
- Allergic reactions: rashes, photosensiticty etc
- Reversible arthopathy: Get’s into the joints, breaking them?
- Abnormal bone & cartilage formation: you don’t give the drug to children and pregnant women
Resistance
- Altered target: there are chromosomal mutations in the bacterial genes that lowers the affinity for fluoroquinolones.
- Decrease accumulation: due to porin channels and efflux pumps.
Sulphonamides: Inhibition of nucleotide (folic acid) synthesis
General facts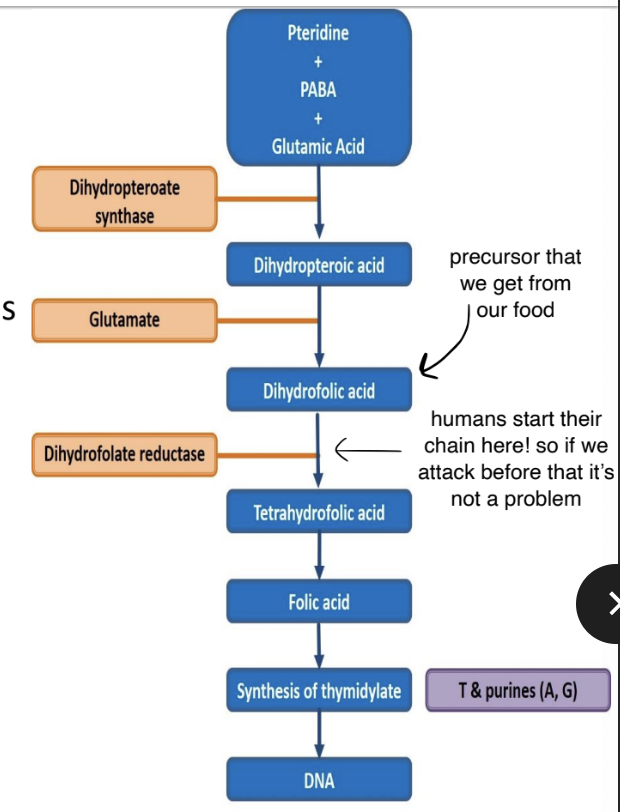
- Folic acids is needed for synthesis of precursors of DNA & RNA in both humans and bacteria.
- Bacteria make their own from PABA (p-amino-benzoix acid)
- Humans used precursors that we take up from our diet
- We are therefore unaffected by anti-folate metabolites
- Sulphonamides have given rise to several important drugs, e.g. acetazolmide
- They are dicided into different groups, depending on if they are short-, intermediate-, or long-lasting.
- Bacteriostatic, but can be bactericidal at higher concentrations
Mechanism of Action
- The sulphonamide part of the molecule resembles PABA.
- It inhibits binding of PABA with dihydropteroate synthase → prevents the forming of dihydropteroic acid (DHF) → inhibits folic acid formation
Pharmacokinetics:
- Administration - Orally, IV, topically (used for bad burns) - can cause reactions
- Absorption - Most cross GIT, reach max concentrations in plasma 4-6hrs
- Distribution - up to 90% bound to albumin, widely distributed, crosses BBB, crosses placenta, reaches inflammatory sites
- Metabolism - acetylated and conjugated primarily in liver
- Excretion - unchanged are eliminated via glomerular filtration & secretion, can be excreted in breast milk
Spectrum of activity
- Baceteriostatic against many G+ and G- bacteria
- E.g. streptococcus and enterobacteria, chlamydia etc
- → stimulates growth of rickettsiae
Clinical use
- Only used in drug combination:
- Urinary tract infections: sulfasalazine
- GIT disorders: Sulfasalazine for IBS :O
Side effects
- GIT: Nausea, vomiting diarreah
- Nephrotoxicity - renal obstruction
- Hypersensiticity reactions - rashes, fever
- Haematological toxicity: anemia
- Kerniciterus: bilirubin induced brain dysfunction
- Hepatotoxicity: jaundice
Resistance
- Many are resistant to sulphonamides
- The resistant bacteria either: overproduce PABA, have low affinity for the DHS enzyme, adopt an alternative pathway in folate metabolism or loss of permeability to sulphonamides.
- When a bacteria becomes resistant to one sulphonamide: it becomes resistant to all :(
Disorganizers of cell membrane structure
The membrane active agents are classified into three groups:
- Affecting membrane structure: e.g. tyrocidine and polymyxins
- Affecting membrane permeability: e.g. valinomycin & nonactin
- Affecting membrane associated enzyme systems
Tyrocidines & Gramicidin A
General facts
- Both contain the amino acid Ornithine which is not found in human proteins.
- Affects membrane structure
Mechanism of Action
- It acts as an ionophore on the bacterial cell wall - meaning that it facilitates ion transport over the membrane.
- It creates a sort of pore in which cl- and Na+ move through.
- This will disrupt the cell homeostasis, and therefore results in bacterial cell death.
Side effects
- Since it is not selective, it will cause toxicity in humans as well
Clinical use
- Primarly used in the treatment of infected surface wounds
Polymyxins
Mechanism of Action
- Binds to lipopolysaccharides in the bacterial membranes: thereby affecting membrane permeability.
- This leads to a displacement of Mg2+ and Ca2+ ions.
- Affects membrane structure (1)
Pharmacokinetics
- Mostly administered intramuscularly for CNS infections due to bad absorption from the gut.
Clinical use & spectrum of activity
- Selective bactericidal activity against Gram negative bacteria, especially for pseudomonas and coliforms.
- Not used that much because highly toxic
Side effects
- Highly toxic
- Causes nephrotoxicity
Inhibitors of cell way synthesis
Beta-lactams
General facts
- There are four categories of beta-lactams:
- Penicillin
- Cephalosporins
- Monobactams
- Carbapenems
- The beta-lactam has a Thiazolidine ring which is its weakness - the bacteria will break this bond which will cause resistance.
Mechanism of Action
- Interfere with synthesis of cell wall component peptidoglycan
- It inhibits the transpeptidation enzyme which cross-links peptide chains attached to the backbone of the peptidoglycan
- Leads to a weaker cell, and therefore cell death
- Is bactericidal
Pharmacokinetics
- AD: Oral, I.V. (for some types), I.M.
- AB: Not great in GIT, food decreases absorption - should be taken on an empty stomach.
- DI: wide, no BBB (except meninges are inflamed),
- ME: metabolism can occur with impaired renal function, oxacillin metabolized in liver.
- EX: Plasma T1/2 < 2 hours, kidney
Spectrum of activity
Is dependent on which type of penicillin:
- Natural penicillin: Against non-beta-lactamase producing G+, e.g. streptococci
- Anti-staphylococcal penicillin: Active against beta-lactamase producing staphylococci
- Aminopenicillins: More active against enterococci and listeria monocytogenes
- Extended-spectrum penicillin: Great activity against G- bacteria, escp. pseudomonas species.
Clinical uses
- Are enormous, but there’s a lot of resistance.
- Examples are: pneumonia, streptococcal, meningitis, UTI’s etc
Side effects
- Hypersensiticty reactions (~10%!)
- GIT disturbances
- Nephritis
- Neurotoxicity: can provoke seizures
- Hematologic toxicity: decreased coagulation
- Some drug intereactions e.g. contraceptive pill
Cephalosporins
General facts
- Related structurally & functionally to penicillins
- Bactericidal
Spectrum of activity
- More active against gram-negative
- They have no activity against LAME - listeria, atypicals (mycoplasma&chlamydia), MRSA and Enterococci.
Pharmacokinetics
- Route of administration: Injection
- Distribution: well distributed, can cross BBB
- Elimination: kidneys
Clinical uses
- pneumonia, sepsis, UTI, meningitis etc
- Treatment of unknown bacteria
- For bacteria which are resistance to beta-lactams
Side effects
- Allergic reactions
- Cross reactivity with penicillin
- GIT disturbances
- Hematological effects (lower prothrombin)
Anti-mycobacterial drugs
Inhibition of cell wall components
Isoniazid
General facts
- Used only for tuberculosis treatment
- Bacteriostatic on resting organisms
- bactericidal for actively growing tubercle bacilli
Mechanism of action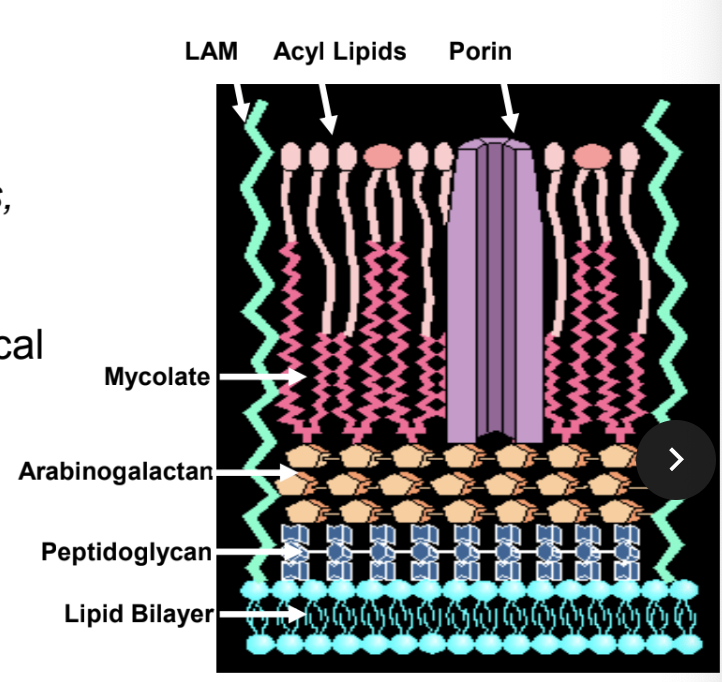
- Inhibits the synthesis of mycolic acids, which are an essential part of mycobacterial cell wall.
- It combines with an enzyme in the mycobacteria leading to disorganization of the metabolism of the cell
- → cell death
Pharmacokinetics
- AD: orally
- AB: very bioavailable
- Distribution: is wide, crosses BBB, 20% bound to plasma proteins
- Metabolism: acetylation
- Excretion: kidneys
Side effects
- Are dose dependant:
- Allergic reactions
- Fever, vasculitis,
- CNS toxicity: memory loss, psychosis, seizures
- Peripheral neuropathy
- Hepatotoxicity
Resistance
- Is normally oxidized in the mycobacterial by catalase-peroxidase which will make it work in them. However, absences of the enzyme will lead to resistance.
- There’s also reduced penetration & over-expression of carrier proteins which leads to resistance.
Ethambutol
Mechanism of action
- Inhibits synthesis of arabinogalactan (part of the cell wall).
Spectrum of activity
- only effective against mycobacteria
- Is sometimes used as a first line anti-tuberculous drug together with isoniaxid, rifampicin and pyrazinamide
Side effects
- are uncommon
- Optic neuritis (is dose related and connected to renal failure)
- GIT dsiturbances
Resistance
- Is common if it’s used alone
- Found in mutations of arabinosyl transgerases
Inhibition of RNA synthesis
Rifampicin
General facts
- bactericidal for mycobacteria
Mechanism of Action
- Binds to beta-subunit of bacterial DNA-dependent RNA polymerase
- Creates a conformational changes that the polymerase have trouble binding to the initiation sequence of DNA
- Thereby it inhibits RNA synthesis
Clinical uses
- As a combination therapy for tuberculosis
- Used for leprosy
Side effects
- Turns urine, tears and sweat orange lol
- Rashes
- Fever
- GIT disturbances
- Jaundice
- Low platelet count
Resistance
- Mutations of RNA polymerase so rifampicin cannot bind