Unit 4 - Chemical attractions
LANGUAGE DEVELOPMENT
polarity - uneven distribution of particles in a molecule —> hydrogen bonding
law of conservation of mass - starting materials = ending materials
chemical equation - reactants and products separated with + signs and the production of new substances with —>
mole - 6.022 × 10²³ particles
solution - solute + solvent
solubility - ability of a solute to dissolve in a solvent
concentration - amount of substance in a defined space
4.1 - properties of materials
exploration 1 - observing properties of compounds
ideas
materials have no overall charge because they have equal numbers of protons and electrons
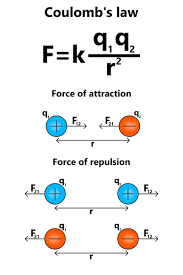
electric force/Coulomb force - repulsions + attractions due to electric charge
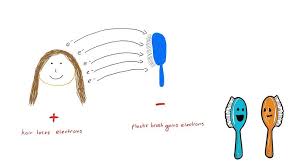
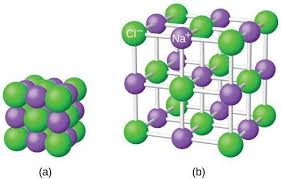
ionic compounds - strong attractive forces hold ions tightly together —> high melting point
molecular compounds - attractive forces still exist —> low melting point
uneven molecular charges
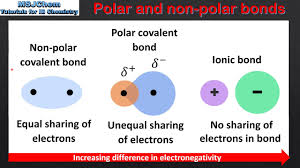
electronegativity - tendency of an atom to pull electrons towards itself
nonpolar covalent bond - two atoms from the same element form a covalent bond, evenly sharing electrons
polarity - uneven distribution of particles in a molecule
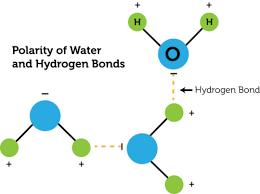
polar molecule - a partial negative charge on one end, and a partial positive charge on the other —> poles
partial charge - unequal sharing (covalent bonds)
dipole - molecule w/ two poles
diatomic - having 2 atoms
2+ atoms - polarity is determined by polarity of individual bonds + 3D arrangement of molecules
3D arrangement - H2O molecule has 2 dipoles. Since it is bent, the arrangement isn’t symmetrical. —> water is highly polar b/c there are 2 unshared electrons on the oxygen atom
negative center of charge - around oxygen atom
positive center of charge - between 2 hydrogen atoms
BOTH RESULT IN HIGH SURFACE TENSION + BOILING POINT
dipole-dipole forces
heat = energy added to a system
KE increases in a liquid’s molecules and they move faster
boiling point - molecules move fast enough to overcome attractive forces between molecules —> pulling away from each other entering gas state
stronger forces are between molecules = higher boiling point
ICl is a polar molecule, whereas Br2 is nonpolar. The boiling point of ICl is likely to be higher than the boiling point of Br2. This is due to dipole-dipole interactions between positive and negative portions of polar molecules.
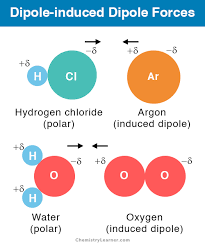
dipole-induced dipole - attraction is a weak attraction that occurs when a polar molecule causes a dipole to form in an atom or nonpolar molecule by disrupting the electron configuration in the nonpolar atom.
A dipole-induced dipole interaction is much weaker than a dipole-dipole interaction because the electrons in the nonpolar atom are shifted to one side of the nucleus in a way that is not sustainable. The shift lasts for only an instant because electrons are in constant motion. —> needs to be balanced?
hydrogen bonding
hydrogen compounds have unusually high boiling points
bonded to a highly electronegative atom —> pulls electron almost completely away—> strong partial positive charge —> polar
ATTRACTION BETWEEN MOLECULES WITH HYDROGEN IN IT!!!
properties of water
relatively small mass/size
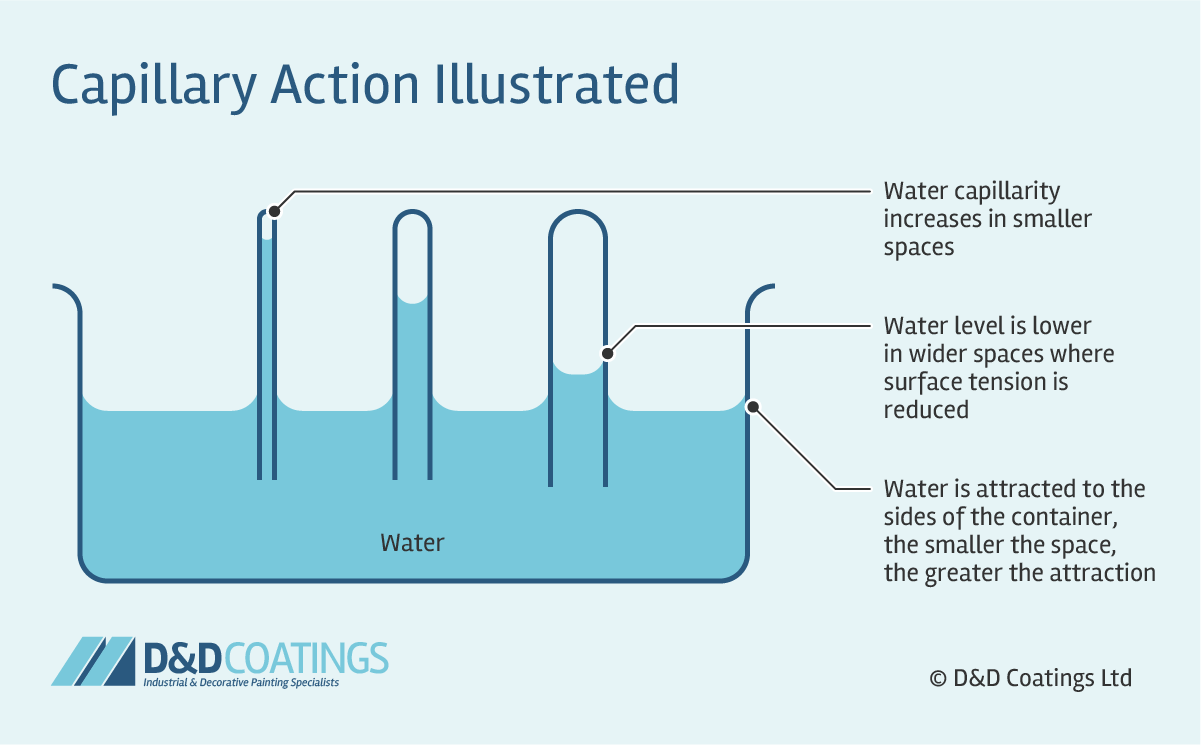
surface tension
climbs up tube - CAPILLARY ACTION
bubble shape —> cohesion between molecules
expands when frozen —> loses KE = more bonds forming —> fixed distance
liquid—>gas = broken bond
The amount of space between molecules in solid water is GREATER than that of liquid water. Density of solid water is LESS than that of liquid water. Substances with lower density FLOAT in substances with higher density.
London dispersion forces
electrons = constant motion
slightly uneven distribution
temporary = + pole/ - pole
induces dipoles in nearby atoms
weak dipoles = constant motion
LONDON DISPERSION FORCE
exists in every type of atom —> also nonpolar
increases with atomic/molar mass
halogens
light = fluorine/chlorine = gases at room temp
large = bromine = liquid
largest = iodine = solid
exploration 3 - materials science and design
structure of materials
materials science - scientific study of properties/applications of materials
alloys - combining different metals that have different properties from their components
metallic bonding - valence electrons shared by the entire solid
thermal/electric conductivity, malleability, ductility, reflect light from shiny surfaces
metal ions exist in a sea of electrons
electrons = free to move between ions
ceramic - not metallic/organic
hard/chemically non-reactive
SOME are conductors
used to make diverse products
semiconductors - electrical conductivity values between conductors and insulators
organic chemist
organic chemistry - field of study focusing on chemistry of carbon-based molecules (like in living things)
structure/function of proteins, carbohydrates, DNA, lipids
how they are produced in the body
molecule interactions
how health is affected
x-ray crystallography
molecular modeling
spectroscopy
* hydrogen bonding if paired with nitrogen/oxygen/fluorine
4.2
exploration 1 - analyzing the composition of matter
Antoine Lavoisier - total amount of matter before/after reaction = the same
law of conservation of mass - starting material = ending material
law of definite proportions - elements are always in a fixed ratio
law of constant composition - chemical compounds contain fixed/constant proportions of their constituent elements
parts of a whole
law of multiple proportions - atoms can rearrange to form new elements
dont break apart
exploration 3 - modeling chemical reactions
chemical equations - reactants and products are separated by +’s and —>’s
balancing chemical equations
coefficients - indicates amount of each reactant/product
multiply subscripts by coefficients —> determine # of atoms
adjust each coefficient until each side is the same
patterns in types of reactions
synthesis - A+B —> AB
decomposition - AB —> A+B
single displacement - A + BD —> AD + B
double displacement - AC + BD —> AD + BC
combustion - CxHx + 02 —> CO2 + H20
chemical energy in the reactants is converted to thermal energy when the fuel is ignited. The fuel reacts with oxygen in the air to form the products carbon dioxide and water. Using combustion engines leads to a increase in the amount of energy stored in earths atmosphere.
exploration 4 - quantifying matter in chemical reactions
mole ratios
2:2
1:2 or 2:1
particles - 6.02 × 10²³ in a mole

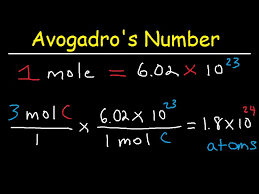
one mole of sugar has a different mass than one mole of salt. The mass of 1 mole of a substance depends on the chemical makeup of the substance. The number of particles in a mole does not depend on the identity of a substance.
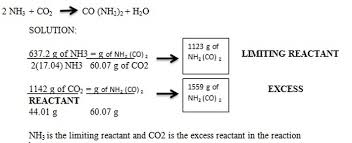
exploration 5 - limiting and excess matter
limit - runs out first
excess - reactant that has extra left over
4.3
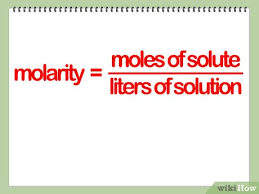
exploration 2 - describing solutions
solution = solvent + solute
homogenous
solvent dissolves the solute
solute - dissolves
solvent - does the dissolving
impacted by: molecular movement
stirring, size, substance its in, temperature, surface area
dissolution - a solute breaks down and mixes uniformly with a solvent
recrystallization - collisions form crystals again
equilibrium - both occur at the same rate
saturated - maximum amount of dissolved solute
unsaturated - increased amount of solvent
solubility - ability of a solute to dissolve in a solvent
temperature/pressure
increased temperature = increased solubility
super satured = unstable
physical disturbance = recrystallization
aqueous solution - solvent is water
universal
polar
hydration - charged parts of water molecule attract/surround positive ions
attract/surround negative ions of the solid
ionic compounds would be generally not soluble in nonpolar solvents. The nonpolar solvents do not have the charges necessary to draw ions out of the crystal into the solution
nonpolar
fats, oils, greases
do not easily dissolve in polar liquids
imiscible = not soluble in each other
miscible = dissolve freely
solubility + pressure
increased = more collisions
more gas dissolves in a liquid
ex: carbonated beverage unopened —> most pressure
solubility + temperature
increases with increasing temperature
more KE
increasing temperature decreases solubility in a gas
dissolving occurs more rapidly
polarity
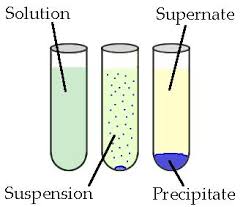
colloids/suspensions
Suspension
heterogenous
large solutes that settle out the solution
ex: muddy water
Colloids
smaller particles than present in suspensions
larger than in a solution
appear homogenous
large enough to scatter light —> Tyndall effect
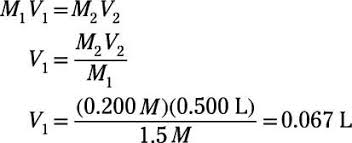
exploration 3 - analyzing the behavior of solutions
dissociation - polar water molecules surround and separate the ions
ionization - polar covalent solute molecules from ions in solution
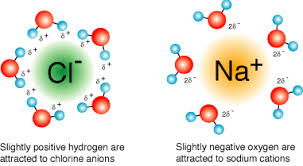
precipitation - mixing results in a combination of ions that form an insoluble compound
attraction between ions > attraction between ions + surrounding water molecules
making pigments, removing salts from water (water treatment), chemical analysis
patterns in solubility
palladium - dental fixtures —> low reacitivity
metal dissolved in chosen solvent (hydrochloric acid)
strong/weak electrolytes
electrolyte - substance conducting electric current when dissolved in solution
yields ions
non electrolyte - does not conduct electric current
does not yield ions
strong: dilute aqeuous solutions conduct electricity well
weak: forms fewer ions in water —> weaker conductivity
sodium chloride conducts electricity well because all of the dissolved compound forms ions. Acetic acid would conduct electricity poorly because some of the dissolved compound forms ions.
colligative properties
pure water cannot conduct electricity
freezes at 0* C —> boils at 100* C
salt water conducts electricity
freezes at a slightly lower temperature and boils at a slightly higher temperature
ex: salt melts icy roads, oceans have salt water so it is not completely solid
dependent on the concentration of solute particles
not dependent on the identity of solute particles
vapor pressure - pressure exerted by a vapor when the vapor is in equilibrium with the liquid or solid form, or both, of the same substance
osmotic pressure - external pressure applied to stop osmosis
dependent on the concentration of solute particles
cells
isotonic - concentration of solute is = into and out of the cell
hypertonic - concentration of solute decreases
water will move out —> shrink
hypotonic - concentration of solute increases
water will move in —> swell
water supply engineer
identify/develop water sources, produce + maintain water purification systems, develop water distribution systems
soluble iron salts - precipitate settles along with particles
chlorine gas - water disinfectant
could cause cancer???
chloramine - disinfectant not forming harmful byproducts
earths water is continually recycled through natural/human designed purification systems
 Knowt
Knowt