TOPIC 3: WHAT A BLAST!
OUTCOME 1: CHEMICAL REACTIONS
Everything is made up of atoms, they are the building blocks of matter. Atoms are made up of smaller particles, known as subatomic particles. This includes a central nucleus (composed of protons and neutrons), as well as one or more electrons that orbit this nucleus.
Protons have a positive charge (pro for positive), neutrons have a neutral charge (neu for neutral), and electrons have a negative charge.
Electrons move rapidly and contain a lot of energy. Since they are of a negative charge and opposite charges attract, they are electrically attracted to the protons in the nucleus which keeps them in orbit.
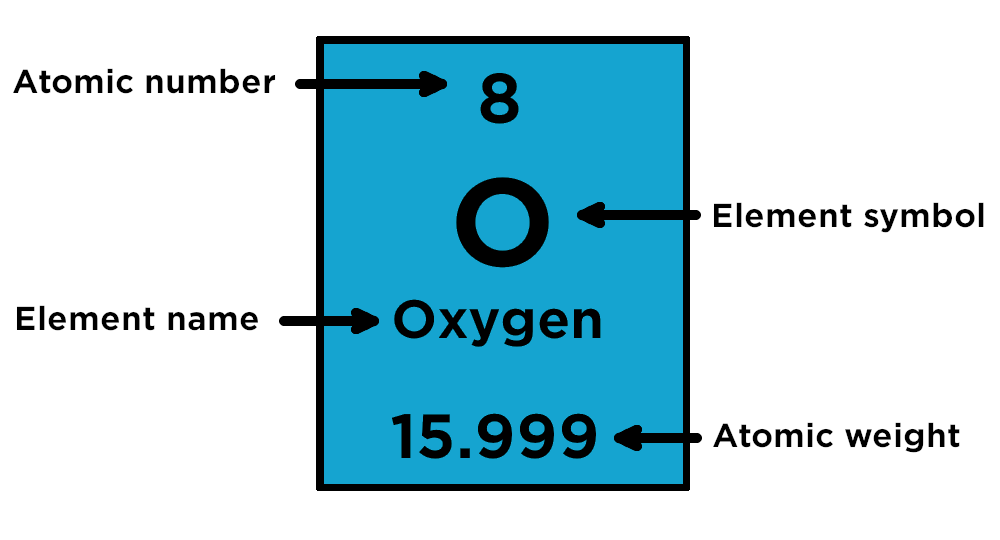
HOW TO READ THE PERIODIC TABLE
The atomic number of an element is the amount of protons in its nucleus. The amount of protons in an atom is equal to the number of electrons in its orbit.
The atomic weight of an element is the amount of protons and neutrons in the atom.
To find the amount of neutrons, subtract the atomic number from the atomic weight.
Periods are read down the periodic table
Groups are read across the periodic table
Chemical reactions occur through rearranging atoms. Matter cannot be created or destroyed. They happen when bonds between atoms are broken and new ones are formed.
Chemical reactions are visible through some observable changes, such as a change in temperature, colour, smell, or the formation of a precipitate or gas.
The substances that react together are called reactants, and the new substances formed are called products.
OUTCOME 2: COMPOUNDS
An element is a pure substance that consists of only one type of atom. A compound, however, is a substance that is formed by two or more types of elements that are chemically bonded. Examples of compounds include:
Water - H2O
Carbon dioxide - CO2
Methane - CH4
Sodium chloride - NaCl
Methane - CH4
Acetic acid - CH3COOH
When atoms react and form a compound, the electrons in the outer shell are most important in order to determine what kind of reaction occurs.
ABOUT IONIC COMPOUNDS
An atom becomes an ion if it gains or loses one or more electrons.
When electrons are lost, they become positively charged. When electrons are gained, they become negatively charged.
Metal atoms tend to lose their electrons, and nonmetal atoms tend to gain them.
When atoms that come into contact with each other lose or gain electrons, it is known as an IONIC COMPOUND. Ionic compounds are formed between metals and nonmetals.
Example: Sodium chloride (NaCl). The sodium atom (Na) loses the electron on its valence (outermost) shell to form a sodium ion, and the chlorine (Cl) atom gains this electron to form a chloride ion (when a non-metal gains an electron, the suffix -ide is added). These oppositely charged (sodium has a positive charge as it is has lost an electron, chlorine has a negative charge as it has gained an electron) ions attract each other (known as an ionic bond) to form the compound sodium chloride.
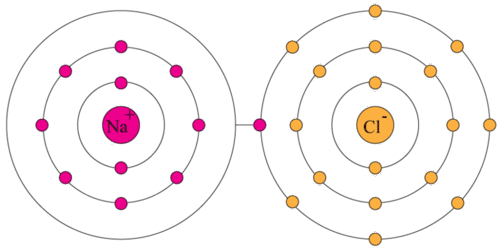
The goal is for both atoms to have a full valence shell. Sometimes, more than two atoms are required to achieve this.
When naming an ionic compound, the names of the two ions are put together, with the metal put first.
Examples: Barium + oxygen = barium oxide, aluminium + sulfur = aluminium sulfide, potassium + chlorine = potassium chloride, etc.
ABOUT COVALENT COMPOUNDS
When non-metal atoms react together, it is known as a covalent compound. Electrons are shared between the atoms in order to achieve a full valence shell and become stable. All elements in the periodic table require 8 electrons for a full outer shell EXCEPT hydrogen, which only needs 2.
Covalent bonds can be single, double, or triple, depending on how many electrons the atoms share.
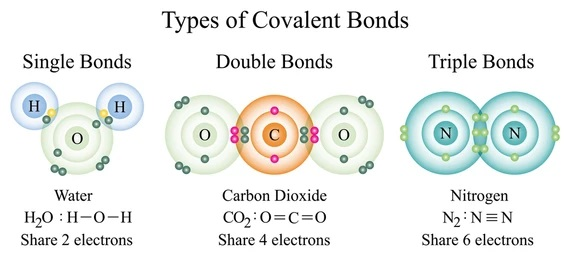
The intermolecular force is weak, and therefore covalent compounds exist in liquids, gases, and solids with low melting points.
There are no free electrons in covalent compounds, so they do not conduct electricity.
ACIDS, BASES, AND SALTS
Acids are corrosive, and react with solid substances such as metal and marble, effectively “eating away” at them.
Strong acids can result in burns and severe injury when they come into contact with skin as they break down proteins and fats found in body tissue.
Bases are soapy or slippery to the touch, and have a bitter taste. Many bases are corrosive, like acids, but some other bases are milder and included in things like baking and cleaning products.
Bases that can be dissolved in water are known as alkalis.

Acids and bases are measured on the pH scale, which ranges from 0-14. A neutral pH is 7. If the pH is low (below 7), it is acidic. If the pH is high (above 7) it is basic. pH is measured by solutions or papers known as indicators which change colour according to the pH level of what is being tested.
Salt is used to describe any product involved in the process of neutralisation that involves bonded positive metal ions and negative non metal ions. There are many kinds of salts that can be produced through different kinds of acids and bases reacting together. Examples of salts include sodium sulfate, magnesium chloride, and sodium acetate.
OUTCOME 3: TYPES OF CHEMICAL REACTIONS
NEUTRALISATION
acid + base —> salt + water
ACIDS AND METALS
acid + metal —> salt + hydrogen
ACIDS AND CARBONATES
acid + metal carbonate —> salt + water + carbon dioxide
CORROSION is the slow reaction of metal with oxygen. NOTE: The term “rust” ONLY applies to iron. Other metals simply corrode. it is what it is
metal + oxygen + water —> metal oxide + energy
COMBUSTION
good lord im boutta combust
When a substance (fuel) reacts with oxygen, it produces energy (generally in the form of heat, light, or sound). Oxygen always has to be present in order for combustion to occur. If there is no oxygen, there is no reaction.
fuel + oxygen —> energy + waste (e.g., carbon dioxide)
PRECIPITATION
A precipitate is an insoluble solid formed during a chemical reaction. Not all reactions will produce a precipitate. An example of this is when lead nitrate and potassium iodide are added together, a yellow solid is produced. This happens when dissolved ions are mixed together and the attraction between the opposite charges are strong enough to create ionic bonds and therefore a new ionic compound.
AB + CD = AC + BD
COMPOSITION AND DECOMPOSITION
Composition is when two reactants form a single product.
It is shown in the form A + B —> AB.
Decomposition is when a single compound breaks down into two simpler chemicals.
It is shown in the form AB —> A + B
OUTCOME 4: CHEMICAL REACTIONS IN BIOLOGICAL SYSTEMS
CELLULAR RESPIRATION
Respiration in a human’s lungs involve a chemical reaction that occurs at a cellular level.
glucose + oxygen —> carbon dioxide + water + energy
PHOTOSYNTHESIS
Photosynthesis is how plants get their “food”. They carry out this process through the chlorophyll present in their cells as well as sunlight.
carbon dioxide + water —> glucose + oxygen
DIGESTION
The stomach contains digestive enzymes and hydrochloric acid which breaks down food into sugars that can be absorbed by the body. Proteins get decomposed into smaller substances known as amino acids.
OUTCOME 5: ENDOTHERMIC AND EXOTHERMIC REACTIONS
The breaking and reforming of bonds during chemical reactions involve transfer, release, A reaction that gives out heat, light, sound, or releases energy is exothermic, “exo”, meaning “outside”, and “therme” meaning “heat”.
Exothermic reactions may be due to the production, crystallisation, or change of states in a substance (e.g., liquid to gas, gas to liquid).
The energy produced in an exothermic reaction is known as thermal energy, which comes from the chemical energy stored in the bonds between the atoms of the reactants. When these bonds are broken, energy is released.
Some exothermic processes are not chemical reactions because no new substances are formed, and instead heat is released through the breaking of the bonds caused by the rearrangement of molecules into new forms/states of matter.
Endothermic reactions, however, absorb energy from their environment in order to make or break chemical bonds between atoms, with endo meaning “internal”.
They reduce temperature of the reacting chemicals as they remove heat from their environment. Like exothermic reactions, endothermic reactions can be both physical or chemical.
Exothermic: surrounding temperature increases
Endothermic: surrounding temperature decreases
OUTCOME 6: RATE OF REACTION IN COMBUSTION REACTIONS
Combustion reactions always involve a fuel combining with oxygen to produce energy. If a combustion reaction is slower, it is known as burning. But if it is faster, it is called an explosion. High temperatures are reached.
To contrast, compare a stove burning to fuel in a car engine. It is the same chemical reaction, just at different rates.
Respiration reactions is a chemical reaction that occurs in the cells of all living beings, both plant and animal. Temperature is controlled.
Combustion and respiration occur at different rates, with respiration being slower than combustion.
OUTCOME 7: CHANGING THE RATE OF CHEMICAL REATIONS
The rate of reaction is the speed at which a chemical reaction proceeds.
Some happen quickly and rapidly, such as combustion, while others are very slow, like corrosion.
Factors that can change the rate of reaction include
Temperature
Concentration
Pressure
Surface area
Catalysts
These factors change the rate of reaction by changing the kinetic energy of the collisions (temperature), number of collisions (concentration, surface area, pressure), or by changing the activation energy (catalysts)
OUTCOME 8: SOCIAL, ETHICAL, ENVIRONMENTAL INFLUENCE OF SCIENCE AND TECHNOLOGY
Enhancing virtual reality
Certain aspects of virtual reality are not developed enough to accurately simulate real world situations.
When using virtual reality as treatment or training, it is important for virtual reality to be able to accurately mimic real life for it to be effective. For example, training a surgeon using virtual reality requires a high amount of accuracy doing a virtual operation, such as when the surgeon is cutting through tissue and needs to be able to feel the different levels of resistance on the scalpel.
Usage of electrorheological fluids can be used to solve problems regarding effectively simulating touch in a virtual environment, as they alter their thickness in response to being exposed to electric fields of different strengths.
OUTCOME 9: ADVANCES IN SCIENCE GENERATING NEW CAREERS
Overheating due to heat from engines or the sun that would heat up the car/room was a large issue, and since air conditioners weren’t a thing yet people had to resolve to building thick walls to control temperature as means of insulation.
Production of plate glass, rolled steel, and mass production in the 1930s allowed for floor to ceiling windows and unobstructed views.
However, as a result, people started to rely on air conditioning in order to stay cool
When buildings got larger, mechanical systems required a lot of energy which led to environmental issues.
Thermo-biometals are a way to resolve this issue, as they require no control and no energy.
It is a ventilation system so that hot air can move through and out where necessary. Shutters and blinds are not needed as it can screen areas and reduce the amount of air conditioning needed inside a particular space.
They do not require energy, and construction and maintenance of them will also open up job and career opportunities as they require engineers and scientists to contribute.